Innovative Metrics for Reporting and Comparing the Glycan Structural Profile in Biotherapeutics
- PMID: 37110538
- PMCID: PMC10143042
- DOI: 10.3390/molecules28083304
Innovative Metrics for Reporting and Comparing the Glycan Structural Profile in Biotherapeutics
Abstract
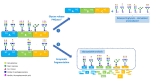
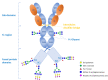
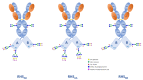
Glycosylation is a critical quality attribute in biotherapeutics, impacting properties such as protein stability, solubility, clearance rate, efficacy, immunogenicity, and safety. Due to the heterogenic and complex nature of protein glycosylation, comprehensive characterization is demanding. Moreover, the lack of standardized metrics for evaluating and comparing glycosylation profiles hinders comparability studies and the establishment of manufacturing control strategies. To address both challenges, we propose a standardized approach based on novel metrics for a comprehensive glycosylation fingerprint which greatly facilitates the reporting and objective comparison of glycosylation profiles. The analytical workflow is based on a liquid chromatography-mass spectrometry-based multi-attribute method. Based on the analytical data, a matrix of glycosylation-related quality attributes, both at site-specific and whole molecule level, are computed, which provide metrics for a comprehensive product glycosylation fingerprint. Two case studies illustrate the applicability of the proposed indices as a standardized and versatile approach for reporting all dimensions of the glycosylation profile. The proposed approach further facilitates the assessments of risks associated with changes in the glycosylation profile that may affect efficacy, clearance, and immunogenicity.
Keywords: N-glycosylation; biosimilarity; biotherapeutics; characterization; glycan analysis; glyco-similarity; glycopeptide mapping; glycosylation index; standardization comparability.
Conflict of interest statement
The authors declare no conflict of interest. At the time of preparation of this manuscript, all authors were employees of subsidiaries of Merck KGaA (Darmstadt, Germany).
Figures

Similar articles
-
Comprehensive analysis and characterization of glycan pairing in therapeutic antibodies and Fc-containing biotherapeutics: Addressing current limitations and implications for N-glycan impact.Eur J Pharm Biopharm. 2024 Jul;200:114325. doi: 10.1016/j.ejpb.2024.114325. Epub 2024 May 15. Eur J Pharm Biopharm. 2024. PMID: 38759899
-
Versatile characterization of glycosylation modification in CTLA4-Ig fusion proteins by liquid chromatography-mass spectrometry.MAbs. 2014;6(6):1474-85. doi: 10.4161/mabs.36313. MAbs. 2014. PMID: 25484062 Free PMC article.
-
Expanding the structural resolution of glycosylation microheterogeneity in therapeutic proteins by salt-free hydrophilic interaction liquid chromatography tandem mass spectrometry.MAbs. 2024 Jan-Dec;16(1):2395503. doi: 10.1080/19420862.2024.2395503. Epub 2024 Aug 27. MAbs. 2024. PMID: 39192481 Free PMC article.
-
Glycan analysis for protein therapeutics.J Chromatogr B Analyt Technol Biomed Life Sci. 2019 Jul 1;1120:29-40. doi: 10.1016/j.jchromb.2019.04.031. Epub 2019 Apr 26. J Chromatogr B Analyt Technol Biomed Life Sci. 2019. PMID: 31063953 Review.
-
N-glycan Analysis in Molecular Medicine: Innovator and Biosimilar Protein Therapeutics.Curr Mol Med. 2020;20(10):828-839. doi: 10.2174/1566524020999201203212352. Curr Mol Med. 2020. PMID: 33272172 Review.
Cited by
-
Advancements in Electrochemical Biosensors for Comprehensive Glycosylation Assessment of Biotherapeutics.Sensors (Basel). 2025 Mar 26;25(7):2064. doi: 10.3390/s25072064. Sensors (Basel). 2025. PMID: 40218579 Free PMC article. Review.
References
-
- Commins S.P., Kelly L.A., Rönmark E., James H.R., Pochan S.L., Peters E.J., Lundbäck B., Nganga L.W., Cooper P.J., Hoskins J.M., et al. Galactose-alpha-1,3-galactose-specific IgE is associated with anaphylaxis but not asthma. Am. J. Respir. Crit. Care Med. 2012;185:723–730. doi: 10.1164/rccm.201111-2017OC. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

