A Review of the Development of Multitarget Molecules against HIV-TB Coinfection Pathogens
- PMID: 37110574
- PMCID: PMC10143421
- DOI: 10.3390/molecules28083342
A Review of the Development of Multitarget Molecules against HIV-TB Coinfection Pathogens
Abstract
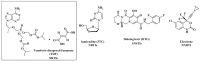
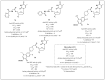
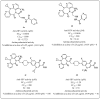
The human immunodeficiency virus (HIV) produces the pathologic basis of acquired immunodeficiency syndrome (AIDS). An increase in the viral load in the body leads to a decline in the number of T lymphocytes, compromising the patient's immune system. Some opportunistic diseases may result, such as tuberculosis (TB), which is the most common in seropositive patients. Long-term treatment is required for HIV-TB coinfection, and cocktails of drugs for both diseases are used concomitantly. The most challenging aspects of treatment are the occurrence of drug interactions, overlapping toxicity, no adherence to treatment and cases of resistance. Recent approaches have involved using molecules that can act synergistically on two or more distinct targets. The development of multitarget molecules could overcome the disadvantages of the therapies used to treat HIV-TB coinfection. This report is the first review on using molecules with activities against HIV and Mycobacterium tuberculosis (MTB) for molecular hybridization and multitarget strategies. Here, we discuss the importance and development of multiple targets as a means of improving adherence to therapy in cases of the coexistence of these pathologies. In this context, several studies on the development of structural entities to treat HIV-TB simultaneously are discussed.
Keywords: AIDS; HIV; coinfection; hybrid; multitarget; tuberculosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






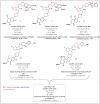
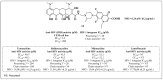
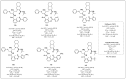

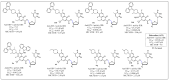




References
-
- UNAIDS [(accessed on 23 February 2023)]. Available online: https://unaids.org.br/estatisticas/#:~:text=As%20novas%20infec%C3%A7%C3%....
Publication types
MeSH terms
Substances
Grants and funding
- 404054/2018-8/National Council for Scientific and Technological Development
- 306193/2018-3/National Council for Scientific and Technological Development
- 311143/2021-0/National Council for Scientific and Technological Development
- 407844/2017-1/National Council for Scientific and Technological Development
- E-26/202.805/2017/Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro
LinkOut - more resources
Full Text Sources
Medical

