Recent Advances in the Synthesis of Propargyl Derivatives, and Their Application as Synthetic Intermediates and Building Blocks
- PMID: 37110613
- PMCID: PMC10146578
- DOI: 10.3390/molecules28083379
Recent Advances in the Synthesis of Propargyl Derivatives, and Their Application as Synthetic Intermediates and Building Blocks
Abstract
The propargyl group is a highly versatile moiety whose introduction into small-molecule building blocks opens up new synthetic pathways for further elaboration. The last decade has witnessed remarkable progress in both the synthesis of propargylation agents and their application in the synthesis and functionalization of more elaborate/complex building blocks and intermediates. The goal of this review is to highlight these exciting advances and to underscore their impact.
Keywords: application in synthesis; catalysts and catalytic systems; homopropargylic reagents; propargylated building blocks and intermediates; propargylating agents; target substrates.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


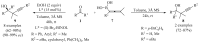
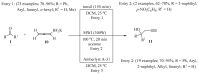









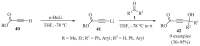




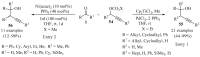






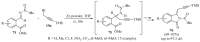
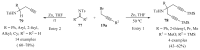
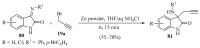



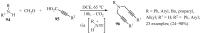




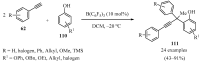
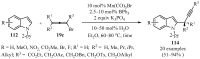


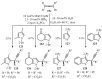

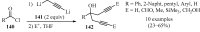






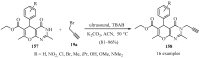

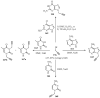
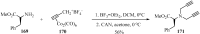











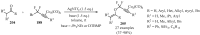



























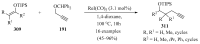





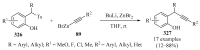







References
-
- Xing Y., Wei Y., Zhou H. Applications of the in situ propargyl-allenyl isomerization in organic synthesis. Curr. Org. Chem. 2012;16:1594–1608. doi: 10.2174/138527212800840973. - DOI
-
- Kobychev V.B., Vitkovskaya N.M., Klyba N.S., Trofimov B.A. Acetylene-allene rearrangement of propargyl systems X-CH2-C≡CH (X = H, Me, NMe2, OMe, F, SMe): An ab initio study. Russ. Chem. Bull. Int. Ed. 2002;51:774–782. doi: 10.1023/A:1016068313892. - DOI
-
- Yamamoto H., Usanov D.L. Comprehensive Organic Synthesis II. 2nd ed. Volume 2. Elsevier; Amsterdam, The Netherlands: 2014. pp. 209–242.
Publication types
LinkOut - more resources
Full Text Sources

