Synthesis and Characterization of Carvedilol-Etched Halloysite Nanotubes Composites with Enhanced Drug Solubility and Dissolution Rate
- PMID: 37110635
- PMCID: PMC10142978
- DOI: 10.3390/molecules28083405
Synthesis and Characterization of Carvedilol-Etched Halloysite Nanotubes Composites with Enhanced Drug Solubility and Dissolution Rate
Abstract
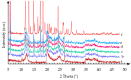
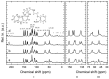
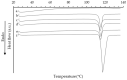
Carvedilol is a poorly water-soluble drug employed to treat chronic heart failure. In this study, we synthesize new carvedilol-etched halloysite nanotubes (HNTs) composites to enhance solubility and dissolution rate. The simple and feasible impregnation method is used for carvedilol loading (30-37% weight). Both the etched HNTs (acidic HCl and H2SO4 and alkaline NaOH treatments) and the carvedilol-loaded samples are characterized by various techniques (XRPD, FT-IR, solid-state NMR, SEM, TEM, DSC, and specific surface area). The etching and loading processes do not induce structural changes. The drug and carrier particles are in intimate contact and their morphology is preserved, as demonstrated by TEM images. The 27Al and 13C solid-state NMR and FT-IR findings show that carvedilol interactions involve the external siloxane surface, especially the aliphatic carbons, the functional groups, and, by inductive effect, the adjacent aromatic carbons. All the carvedilol-halloysite composites display enhanced dissolution rate, wettability, and solubility, as compared to carvedilol. The best performances are obtained for the carvedilol-halloysite system based on HNTs etched with HCl 8M, which exhibits the highest value of specific surface area (91 m2 g-1). The composites make the drug dissolution independent of the environmental conditions of the gastrointestinal tract and its absorption less variable, more predictable, and independent from the pH of the medium.
Keywords: carvedilol; dissolution tests; drug–nanoclay composites; halloysite nanotubes; solid-state NMR.
Conflict of interest statement
The authors declare no conflict of interest.
Figures










References
-
- Good D.J., Rodríguez-Hornedo N. Solubility Advantage of Pharmaceutical Cocrystals. Cryst. Growth Des. 2009;9:2252–2264. doi: 10.1021/cg801039j. - DOI
-
- Kumar S., Singh P. Various Techniques for Solubility Enhancement: An Overview. Pharma Innov. 2016;5:23–28.
-
- Braga D., Grepioni F., Maini L., Polito M. Crystal Polymorphism and Multiple Crystal Forms. In: Hosseini M.W., editor. Molecular Networks. Springer; Berlin/Heidelberg, Germany: 2009. pp. 87–95. Structure and Bonding.
-
- Lu J., Li Z., Jiang X. Polymorphism of Pharmaceutical Molecules: Perspectives on Nucleation. Front. Chem. Sci. Eng. 2010;4:37–44. doi: 10.1007/s11705-009-0294-2. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

