Identification of Novel Cyclooxygenase-1 Selective Inhibitors of Thiadiazole-Based Scaffold as Potent Anti-Inflammatory Agents with Safety Gastric and Cytotoxic Profile
- PMID: 37110650
- PMCID: PMC10142904
- DOI: 10.3390/molecules28083416
Identification of Novel Cyclooxygenase-1 Selective Inhibitors of Thiadiazole-Based Scaffold as Potent Anti-Inflammatory Agents with Safety Gastric and Cytotoxic Profile
Abstract
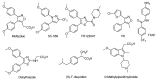
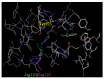
Major obstacles faced by the use of nonsteroidal anti-inflammatory drugs (NSAID) are their gastrointestinal toxicity induced by non-selective inhibition of both cyclooxygenases (COX) 1 and 2 and their cardiotoxicity associated with a certain class of COX-2 selective inhibitors. Recent studies have demonstrated that selective COX-1 and COX-2 inhibition generates compounds with no gastric damage. The aim of the current study is to develop novel anti-inflammatory agents with a better gastric profile. In our previous paper, we investigated the anti-inflammatory activity of 4-methylthiazole-based thiazolidinones. Thus, based on these observations, herein we report the evaluation of anti-inflammatory activity, drug action, ulcerogenicity and cytotoxicity of a series of 5-adamantylthiadiazole-based thiazolidinone derivatives. The in vivo anti-inflammatory activity revealed that the compounds possessed moderate to excellent anti-inflammatory activity. Four compounds 3, 4, 10 and 11 showed highest potency (62.0, 66.7, 55.8 and 60.0%, respectively), which was higher than the control drug indomethacin (47.0%). To determine their possible mode of action, the enzymatic assay was conducted against COX-1, COX-2 and LOX. The biological results demonstrated that these compounds are effective COX-1 inhibitors. Thus, the IC50 values of the three most active compounds 3, 4 and 14 as COX-1 inhibitors were 1.08, 1.12 and 9.62 μΜ, respectively, compared to ibuprofen (12.7 μΜ) and naproxen (40.10 μΜ) used as control drugs. Moreover, the ulcerogenic effect of the best compounds 3, 4 and 14 were evaluated and revealed that no gastric damage was observed. Furthermore, compounds were found to be nontoxic. A molecular modeling study provided molecular insight to rationalize the COX selectivity. In summary, we discovered a novel class of selective COX-1 inhibitors that could be effectively used as potential anti-inflammatory agents.
Keywords: anti-inflammatory; cyclooxygenase; cytotoxicity; enzyme inhibition; lipoxygenase; molecular modeling; thiadiazole; ulcerogenic effect.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








Similar articles
-
Synthesis, biological evaluation and docking study of 1,3,4-thiadiazole-thiazolidinone hybrids as anti-inflammatory agents with dual inhibition of COX-2 and 15-LOX.Bioorg Chem. 2018 Oct;80:461-471. doi: 10.1016/j.bioorg.2018.06.036. Epub 2018 Jul 3. Bioorg Chem. 2018. PMID: 29986191
-
Synthesis and biological evaluation of new nicotinate derivatives as potential anti-inflammatory agents targeting COX-2 enzyme.Bioorg Chem. 2021 Feb;107:104610. doi: 10.1016/j.bioorg.2020.104610. Epub 2021 Jan 5. Bioorg Chem. 2021. PMID: 33454504
-
Conjugation of 4-aminosalicylate with thiazolinones afforded non-cytotoxic potent in vitro and in vivo anti-inflammatory hybrids.Bioorg Chem. 2020 Jan;94:103378. doi: 10.1016/j.bioorg.2019.103378. Epub 2019 Oct 24. Bioorg Chem. 2020. PMID: 31677858
-
Dual acting anti-inflammatory drugs: a reappraisal.Pharmacol Res. 2001 Dec;44(6):437-50. doi: 10.1006/phrs.2001.0872. Pharmacol Res. 2001. PMID: 11735348 Review.
-
Evolution of nonsteroidal anti-inflammatory drugs (NSAIDs): cyclooxygenase (COX) inhibition and beyond.J Pharm Pharm Sci. 2008 Sep 20;11(2):81s-110s. doi: 10.18433/j3t886. J Pharm Pharm Sci. 2008. PMID: 19203472 Review.
Cited by
-
Pharmacovigilance analysis of small bowel bleeding associated with NSAIDs.Ther Adv Drug Saf. 2025 May 6;16:20420986251318848. doi: 10.1177/20420986251318848. eCollection 2025. Ther Adv Drug Saf. 2025. PMID: 40336902 Free PMC article.
-
Carborane-Based Analogs of Celecoxib and Flurbiprofen, their COX Inhibition Potential, and COX Selectivity Index.ChemMedChem. 2025 Jun 2;20(11):e202500166. doi: 10.1002/cmdc.202500166. Epub 2025 May 4. ChemMedChem. 2025. PMID: 40128115 Free PMC article.
References
-
- da Silva T.L., Costa C.S.D., da Silva M.G.C., Vieira M.G.A. Overview of non-steroidal anti-inflammatory drugs degradation by advanced oxidation processes. J. Clean. Prod. 2022;346:131226. doi: 10.1016/j.jclepro.2022.131226. - DOI
-
- Batchu S.N., Chaudhary K., Zlobine I., Pawa J., Seubert J.M. Handbook of Lipids in Human Function. Elsevier; Amsterdam, The Netherlands: 2016. Fatty Acids and Cardiac Ischemia Reperfusion Injury; pp. 39–83.
-
- Stone W.L., Basit H., Burns B. StatPearls [Internet] StatPearls Publishing; Treasure Island, FL, USA: 2021. Pathology, Inflammation. - PubMed
-
- Silverstein F.E., Faich G., Goldstein J.L., Simon L.S., Pincus T., Whelton A., Makuch R., Eisen G., Agrawal N.M., Stenson W.F., et al. Gastrointestinal Toxicity With Celecoxib vs Nonsteroidal Anti-inflammatory Drugs for Osteoarthritis and Rheumatoid ArthritisThe CLASS Study: A Randomized Controlled Trial. JAMA. 2000;284:1247–1255. doi: 10.1001/jama.284.10.1247. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

