Photoinduced Dynamics of 13,13'-Diphenylpropyl-β-carotene
- PMID: 37110738
- PMCID: PMC10143239
- DOI: 10.3390/molecules28083505
Photoinduced Dynamics of 13,13'-Diphenylpropyl-β-carotene
Abstract
Carotenoids are ubiquitous pigment systems in nature which are relevant to a range of processes, such as photosynthesis, but the detailed influence of substitutions at the polyene backbone on their photophysics is still underexplored. Here, we present a detailed experimental and theoretical investigation of the carotenoid 13,13'-diphenylpropyl-β-carotene using ultrafast transient absorption spectroscopy and steady-state absorption experiments in n-hexane and n-hexadecane, complemented by DFT/TDDFT calculations. In spite of their bulkiness and their potential capability to "fold back" onto the polyene system, which could result in π-stacking effects, the phenylpropyl residues have only a minor impact on the photophysical properties compared with the parent compound β-carotene. Ultrafast spectroscopy finds lifetimes of 200-300 fs for the S2 state and 8.3-9.5 ps for the S1 state. Intramolecular vibrational redistribution with time constants in the range 0.6-1.4 ps is observed in terms of a spectral narrowing of the S1 spectrum over time. We also find clear indications of the presence of vibrationally hot molecules in the ground electronic state (S0*). The DFT/TDDFT calculations confirm that the propyl spacer electronically decouples the phenyl and polyene π-systems and that the substituents in the 13 and 13' positions point away from the polyene system.
Keywords: DFT/TDDFT calculations; carotenoids; ultrafast laser spectroscopy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


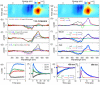


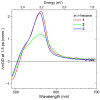
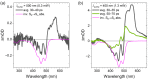


References
-
- Frank H.A., Young A.J., Britton G., Cogdell R.J. The Photochemistry of Carotenoids. Volume 8. Kluwer; Dordrecht, The Netherlands: 1999. p. 399.
-
- Ehlers F., Scholz M., Lenzer T., Oum K. Excited State Dynamics of Selected All-trans C40 Xanthophyll Carotenoids. Z. Phys. Chem. 2015;229:1815–1830. doi: 10.1515/zpch-2015-0605. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

