Rational Design of Disulfide Bridges in Bb PETaseCD for Enhancing the Enzymatic Performance in PET Degradation
- PMID: 37110762
- PMCID: PMC10146679
- DOI: 10.3390/molecules28083528
Rational Design of Disulfide Bridges in Bb PETaseCD for Enhancing the Enzymatic Performance in PET Degradation
Abstract
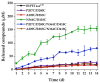
Polyethylene terephthalate (PET) is one of the most prevalent transparent thermoplastics. It is commonly utilized due to its low cost and high durability. With the massive accumulation of waste PET, however, serious environmental pollution has become a global problem. Compared to traditional chemical degradation, biodegradation of PET catalyzed by PET hydrolase (PETase) is more environmentally friendly and energy-efficient. BbPETaseCD from the Burkholderiales bacterium is a PETase that shows favorable properties for application in the biodegradation of PET. To enhance the enzymatic performance of this enzyme, this work focuses on the rational design of disulfide bridges in BbPETaseCD. We utilized two computational algorithms to predict the probable disulfide-bridge mutations in BbPETaseCD, and five variants were acquired from the computations. Among these, the N364C/D418C variant with one additional disulfide bond showed higher expression than the wild-type enzyme (WT) and the best enzymatic performance. The melting temperature (Tm) of the N364C/D418C variant presented an increase of 14.8 °C over that of WT (56.5 °C), indicating that the additional disulfide bond significantly raised the thermodynamic stability of the enzyme. Kinetic experiments at different temperatures also demonstrated the thermal stability increase of the variant. The variant also showed significantly increased activity over WT when using bis(hydroxyethyl) terephthalate (BHET) as the substrate. More remarkably, the N364C/D418C variant exhibited approximately an 11-fold increase over the WT enzyme in the long-term (14 days) degradation of PET films. The results prove that the rationally designed disulfide bond significantly improved the enzymatic performance of the enzyme for PET degradation.
Keywords: BbPETaseCD; PET degradation; disulfide bridge; rational design.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Li C., Ban X., Zhang Y., Gu Z., Hong Y., Cheng L., Tang X., Li Z. Rational design of disulfide bonds for enhancing the thermostability of the 1,4-α-glucan branching enzyme from Geobacillus thermoglucosidans STB02. J. Agric. Food Chem. 2020;68:13791–13797. doi: 10.1021/acs.jafc.0c04798. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

