A Glutamate Scavenging Protocol Combined with Deanna Protocol in SOD1-G93A Mouse Model of ALS
- PMID: 37111040
- PMCID: PMC10141074
- DOI: 10.3390/nu15081821
A Glutamate Scavenging Protocol Combined with Deanna Protocol in SOD1-G93A Mouse Model of ALS
Abstract
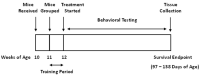
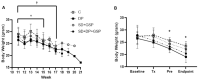
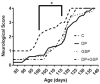
Amyotrophic lateral sclerosis (ALS) is a progressive disease of neuronal degeneration in the motor cortex, brainstem, and spinal cord, resulting in impaired motor function and premature demise as a result of insufficient respiratory drive. ALS is associated with dysfunctions in neurons, neuroglia, muscle cells, energy metabolism, and glutamate balance. Currently, there is not a widely accepted, effective treatment for this condition. Prior work from our lab has demonstrated the efficacy of supplemental nutrition with the Deanna Protocol (DP). In the present study, we tested the effects of three different treatments in a mouse model of ALS. These treatments were the DP alone, a glutamate scavenging protocol (GSP) alone, and a combination of the two treatments. Outcome measures included body weight, food intake, behavioral assessments, neurological score, and lifespan. Compared to the control group, DP had a significantly slower decline in neurological score, strength, endurance, and coordination, with a trend toward increased lifespan despite a greater loss of weight. GSP had a significantly slower decline in neurological score, strength, endurance, and coordination, with a trend toward increased lifespan. DP+GSP had a significantly slower decline in neurological score with a trend toward increased lifespan, despite a greater loss of weight. While each of the treatment groups fared better than the control group, the combination of the DP+GSP was not better than either of the individual treatments. We conclude that the beneficial effects of the DP and the GSP in this ALS mouse model are distinct, and appear to offer no additional benefit when combined.
Keywords: CoQ10; Deanna Protocol; amyotrophic lateral sclerosis; blood glutamate scavengers; medium chain triglycerides; motor neuron disease; neurodegeneration; oxaloacetic acid.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of this study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures





References
-
- Halperin J.J., Kaplan G.P., Brazinsky S., Tsai T.F., Cheng T., Ironside A., Wu P., Delfiner J., Golightly M., Brown R.H., et al. Immunologic reactivity against Borrelia burgdorferi in patients with motor neuron disease. Arch. Neurol. 1990;47:586–594. doi: 10.1001/archneur.1990.00530050110021. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

