High-Dose Spermidine Supplementation Does Not Increase Spermidine Levels in Blood Plasma and Saliva of Healthy Adults: A Randomized Placebo-Controlled Pharmacokinetic and Metabolomic Study
- PMID: 37111071
- PMCID: PMC10143675
- DOI: 10.3390/nu15081852
High-Dose Spermidine Supplementation Does Not Increase Spermidine Levels in Blood Plasma and Saliva of Healthy Adults: A Randomized Placebo-Controlled Pharmacokinetic and Metabolomic Study
Abstract
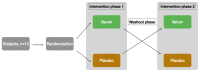
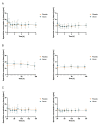
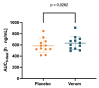
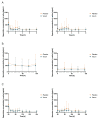
(1) Background: Spermidine is a biogenic polyamine that plays a crucial role in mammalian metabolism. As spermidine levels decline with age, spermidine supplementation is suggested to prevent or delay age-related diseases. However, valid pharmacokinetic data regarding spermidine remains lacking. Therefore, for the first time, the present study investigated the pharmacokinetics of oral spermidine supplementation. (2) Methods: This study was designed as a randomized, placebo-controlled, triple-blinded, two-armed crossover trial with two 5-day intervention phases separated by a washout phase of 9 days. In 12 healthy volunteers, 15 mg/d of spermidine was administered orally, and blood and saliva samples were taken. Spermidine, spermine, and putrescine were quantified by liquid chromatography-mass spectrometry (LC-MS/MS). The plasma metabolome was investigated using nuclear magnetic resonance (NMR) metabolomics. (3) Results: Compared with a placebo, spermidine supplementation significantly increased spermine levels in the plasma, but it did not affect spermidine or putrescine levels. No effect on salivary polyamine concentrations was observed. (4) Conclusions: This study's results suggest that dietary spermidine is presystemically converted into spermine, which then enters systemic circulation. Presumably, the in vitro and clinical effects of spermidine are at least in part attributable to its metabolite, spermine. It is rather unlikely that spermidine supplements with doses <15 mg/d exert any short-term effects.
Keywords: COVID-19; SARS-CoV-2; autophagy; putrescine; spermidine; spermine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Zou D., Zhao Z., Li L., Min Y., Zhang D., Ji A., Jiang C., Wei X., Wu X. A Comprehensive Review of Spermidine: Safety, Health Effects, Absorption and Metabolism, Food Materials Evaluation, Physical and Chemical Processing, and Bioprocessing. Comp. Rev. Food Sci. Food Safe. 2022;21:2820–2842. doi: 10.1111/1541-4337.12963. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

