Antidiabetic Activity of Potential Probiotics Limosilactobacillus spp., Levilactobacillus spp., and Lacticaseibacillus spp. Isolated from Fermented Sugarcane Juice: A Comprehensive In Vitro and In Silico Study
- PMID: 37111101
- PMCID: PMC10144524
- DOI: 10.3390/nu15081882
Antidiabetic Activity of Potential Probiotics Limosilactobacillus spp., Levilactobacillus spp., and Lacticaseibacillus spp. Isolated from Fermented Sugarcane Juice: A Comprehensive In Vitro and In Silico Study
Abstract
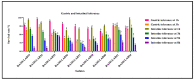
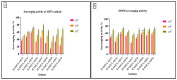
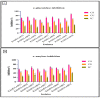
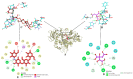
Probiotics are regarded as a potential source of functional foods for improving the microbiota in human gut. When consumed, these bacteria can control the metabolism of biomolecules, which has numerous positive effects on health. Our objective was to identify a probiotic putative Lactobacillus spp. from fermented sugarcane juice that can prevent α-glucosidase and α-amylase from hydrolyzing carbohydrates. Isolates from fermented sugarcane juice were subjected to biochemical, molecular characterization (16S rRNA) and assessed for probiotic traits. Cell-free supernatant (CS) and extract (CE) and also intact cells (IC) were examined for the inhibitory effect on α-glucosidase and α-amylase. CS of the strain showed the highest inhibition and was subjected to a liquid chromatography-mass spectrometry (LCMS) analysis to determine the organic acid profile. The in silico approach was employed to assess organic acid stability and comprehend enzyme inhibitors' impact. Nine isolates were retained for further investigation based on the preliminary biochemical evaluation. Limosilactobacillus spp., Levilactobacillus spp., and Lacticaseibacillus spp. were identified based on similarity > 95% in homology search (NCBI database). The strains had a higher survival rate (>98%) than gastric and intestinal fluids, also a high capacity for adhesion (hydrophobicity > 56%; aggregation > 80%; HT-29 cells > 54%; buccal epithelial cells > 54%). The hemolytic assay indicated that the isolates could be considered safe. The isolates' derivatives inhibited enzymes to varying degrees, with α-glucosidase inhibition ranging from 21 to 85% and α-amylase inhibition from 18 to 75%, respectively. The CS of RAMULAB54 was profiled for organic acid that showed the abundance of hydroxycitric acid, citric acid, and lactic acid indicating their role in the observed inhibitory effects. The in silico approach has led us to understand that hydroxycitric acid has the ability to inhibit both the enzymes (α-glucosidase and α-amylase) effectively. Inhibiting these enzymes helps moderate postprandial hyperglycemia and regulates blood glucose levels. Due to their promising antidiabetic potential, these isolates can be used to enhance intestinal health.
Keywords: lactic acid bacteria; probiotic; sugarcane; α-amylase; α-glucosidase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Ramu R., Shirahatti P.S., Zameer F., Ranganatha L.V., Nagendra Prasad M.N. Inhibitory Effect of Banana (Musa Sp. Var. Nanjangud Rasa Bale) Flower Extract and Its Constituents Umbelliferone and Lupeol on α-Glucosidase, Aldose Reductase and Glycation at Multiple Stages. S. Afr. J. Bot. 2014;95:54–63. doi: 10.1016/j.sajb.2014.08.001. - DOI
-
- Sarwar N., Gao P., Kondapally Seshasai S.R., Gobin R., Kaptoge S., di Angelantonio E., Ingelsson E., Lawlor D.A., Selvin E., Stampfer M., et al. Diabetes Mellitus, Fasting Blood Glucose Concentration, and Risk of Vascular Disease: A Collaborative Meta-Analysis of 102 Prospective Studies. Lancet. 2010;375:2215–2222. doi: 10.1016/S0140-6736(10)60484-9. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

