Effect of Lacticaseibacillus paracasei N1115 on Immunomodulatory and Gut Microbial Composition in Young Children: A Randomized, Placebo-Controlled Study
- PMID: 37111189
- PMCID: PMC10145370
- DOI: 10.3390/nu15081970
Effect of Lacticaseibacillus paracasei N1115 on Immunomodulatory and Gut Microbial Composition in Young Children: A Randomized, Placebo-Controlled Study
Abstract
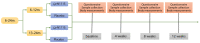
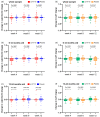
Lactobacillus paracasei N1115 (Lp N1115) was isolated from fermented milk products. The administration of Lp N1115 is safe and well tolerated in Chinese children, but its effectiveness among young Chinese children is still unclear. To investigate the efficacy of Lp N1115 as a probiotic to enhance gut development in Chinese infants and toddlers born by cesarean section, 109 healthy and cesarean-delivered infants aged 6-24 months were recruited for a 12-week randomized, placebo-controlled trial, with 101 finally completing the study. Saliva and stool samples were collected and detected at weeks 0, 4, 8, and 12 of the intervention. Statistical analyses were performed by using a per-protocol (PP) approach. After 12 weeks of intervention, the fecal pH in the control group increased (p = 0.003), while the fecal pH in the experimental group did not change. Salivary cortisol decreased from baseline in the experimental group (p = 0.023), while the control group showed little change. In addition, Lp N1115 increased the fecal sIgA content of infants aged 6-12 months (p = 0.044) but had no obvious effect on fecal calprotectin and saliva sIgA. At week 4, the increase in Lactobacillus relative to baseline was higher in the experimental group than in the control group (p = 0.019). Further analysis showed a trend toward a higher detection rate of Lactobacillus in the experimental group than in the control group (p = 0.039). In conclusion, Lp N1115 was able to enhance the content of Lactobacillus and maintain fecal pH levels. Its beneficial effects on gut development were more obvious in 6-12-month-old infants.
Keywords: Lacticaseibacillus; cesarean section; probiotic; young children.
Conflict of interest statement
S. Wang, Y. Xun, and Q. Yuan are employed by Shijiazhuang Junlebao Dairy Co., Ltd. and cooperated in designing the project. The authors declare no other conflict of interest.
Figures




References
-
- Miller J.E., Goldacre R., Moore H.C., Zeltzer J., Knight M., Morris C., Nowell S., Wood R., Carter K.W., Fathima P., et al. Mode of birth and risk of infection-related hospitalisation in childhood: A population cohort study of 7.17 million births from 4 high-income countries. PLoS Med. 2020;17:e1003429. doi: 10.1371/journal.pmed.1003429. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

