Asialo-rhuEPO as a Potential Neuroprotectant for Ischemic Stroke Treatment
- PMID: 37111367
- PMCID: PMC10143832
- DOI: 10.3390/ph16040610
Asialo-rhuEPO as a Potential Neuroprotectant for Ischemic Stroke Treatment
Abstract
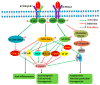
Neuroprotective drugs to protect the brain against cerebral ischemia and reperfusion (I/R) injury are urgently needed. Mammalian cell-produced recombinant human erythropoietin (rhuEPOM) has been demonstrated to have excellent neuroprotective functions in preclinical studies, but its neuroprotective properties could not be consistently translated in clinical trials. The clinical failure of rhuEPOM was thought to be mainly due to its erythropoietic activity-associated side effects. To exploit its tissue-protective property, various EPO derivatives with tissue-protective function only have been developed. Among them, asialo-rhuEPO, lacking terminal sialic acid residues, was shown to be neuroprotective but non-erythropoietic. Asialo-rhuEPO can be prepared by enzymatic removal of sialic acid residues from rhuEPOM (asialo-rhuEPOE) or by expressing human EPO gene in glycoengineered transgenic plants (asialo-rhuEPOP). Both types of asialo-rhuEPO, like rhuEPOM, displayed excellent neuroprotective effects by regulating multiple cellular pathways in cerebral I/R animal models. In this review, we describe the structure and properties of EPO and asialo-rhuEPO, summarize the progress on neuroprotective studies of asialo-rhuEPO and rhuEPOM, discuss potential reasons for the clinical failure of rhuEPOM with acute ischemic stroke patients, and advocate future studies needed to develop asialo-rhuEPO as a multimodal neuroprotectant for ischemic stroke treatment.
Keywords: asialo-erythropoietin; cerebral ischemia and reperfusion; clinical trial; erythropoietin; erythropoietin receptor; hematopoietic activity; multimodal neuroprotectant; non-erythropoiesis; preclinical study.
Conflict of interest statement
J.X., F.K. and C.-Y.H. are inventors of filed patent “Methods for the production of cytoprotective asialo-erythropoietin in plants and its purification from plant tissues” (PCT NUMBER: US2013031382, pending).
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

