Leishmania Infection-Induced Proteolytic Processing of SIRPα in Macrophages
- PMID: 37111479
- PMCID: PMC10146913
- DOI: 10.3390/pathogens12040593
Leishmania Infection-Induced Proteolytic Processing of SIRPα in Macrophages
Abstract
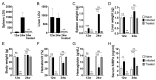
The shedding of cell surface receptors may bring synergistic outcomes through the loss of receptor-mediated cell signaling and competitive binding of the shed soluble receptor to its ligand. Thus, soluble receptors have both biological importance and diagnostic importance as biomarkers in immunological disorders. Signal regulatory protein α (SIRPα), one of the receptors responsible for the 'don't-eat-me' signal, is expressed by myeloid cells where its expression and function are in part regulated by proteolytic cleavage. However, reports on soluble SIRPα as a biomarker are limited. We previously reported that mice with experimental visceral leishmaniasis (VL) manifest anemia and enhanced hemophagocytosis in the spleen accompanied with decreased SIRPα expression. Here, we report increased serum levels of soluble SIRPα in mice infected with Leishmania donovani, a causative agent of VL. Increased soluble SIRPα was also detected in a culture supernatant of macrophages infected with L. donovani in vitro, suggesting the parasite infection promotes ectodomain shedding of SIRPα on macrophages. The release of soluble SIRPα was partially inhibited by an ADAM proteinase inhibitor in both LPS stimulation and L. donovani infection, suggesting a shared mechanism for cleavage of SIRPα in both cases. In addition to the ectodomain shedding of SIRPα, both LPS stimulation and L. donovani infection induced the loss of the cytoplasmic region of SIRPα. Although the effects of these proteolytic processes or changes in SIRPα still remain unclear, these proteolytic regulations on SIRPα during L. donovani infection may explain hemophagocytosis and anemia induced by infection, and serum soluble SIRPα may serve as a biomarker for hemophagocytosis and anemia in VL and the other inflammatory disorders.
Keywords: ADAM; SIRPα; ectodomain shedding; visceral leishmaniasis.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of this study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources

