Platinum(IV)-Loaded Degraded Glycol Chitosan as Efficient Platinum(IV) Drug Delivery Platform
- PMID: 37111536
- PMCID: PMC10145531
- DOI: 10.3390/pharmaceutics15041050
Platinum(IV)-Loaded Degraded Glycol Chitosan as Efficient Platinum(IV) Drug Delivery Platform
Abstract
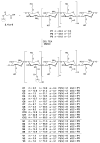
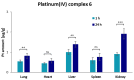
A new class of anticancer prodrugs was designed by combining the cytotoxicity of platinum(IV) complexes and the drug carrier properties of glycol chitosan polymers: Unsymmetrically carboxylated platinum(IV) analogues of cisplatin, carboplatin and oxaliplatin, namely (OC-6-44)-acetatodiammine(3-carboxypropanoato)dichloridoplatinum(IV), (OC-6-44)-acetaodiammine(3-carboxypropanoato)(cyclobutane-1,1-dicarboxylato)platinum(IV) and (OC-6-44)-acetato(3-carboxypropanoato)(1R,2R-cyclohexane-1,2-diamine)oxalatoplatinum(IV) were synthesised and conjugated via amide bonding to degraded glycol chitosan (dGC) polymers with different chain lengths (5, 10, 18 kDa). The 15 conjugates were investigated with 1H and 195Pt NMR spectroscopy, and average amounts of platinum(IV) units per dGC polymer molecule with ICP-MS, revealing a range of 1.3-22.8 platinum(IV) units per dGC molecule. Cytotoxicity was tested with MTT assays in the cancer cell lines A549, CH1/PA-1, SW480 (human) and 4T1 (murine). IC50 values in the low micromolar to nanomolar range were obtained, and higher antiproliferative activity (up to 72 times) was detected with dGC-platinum(IV) conjugates in comparison to platinum(IV) counterparts. The highest cytotoxicity (IC50 of 0.036 ± 0.005 µM) was determined in CH1/PA-1 ovarian teratocarcinoma cells with a cisplatin(IV)-dGC conjugate, which is hence 33 times more potent than the corresponding platinum(IV) complex and twice more potent than cisplatin. Biodistribution studies of an oxaliplatin(IV)-dGC conjugate in non-tumour-bearing Balb/C mice showed an increased accumulation in the lung compared to the unloaded oxaliplatin(IV) analogue, arguing for further activity studies.
Keywords: anticancer; drug delivery; glycol chitosan; platinum(IV) complexes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Polaris Market Research . Global Platinum Based Cancer Drugs Market Share, Size, Trends, Industry Analysis Report By Drug Type (Cisplatin, Oxaliplatin, Carboplatin, Other), By Application (Colorectal Cancer, Ovarian Cancer, Lung Cancer, Other); By Regions, and Segment Forecast, 2019–2026. Polaris Market Research & Consulting LLP; Pune, India: 2019.
-
- Varbanov H.P., Göschl S., Heffeter P., Theiner S., Roller A., Jensen F., Jakupec M.A., Berger W., Galanski M., Keppler B.K. A Novel Class of Bis-and Tris-Chelate Diam(m)Inebis(Dicarboxylato) Platinum(IV) Complexes as Potential Anticancer Prodrugs. J. Med. Chem. 2014;57:6751–6764. doi: 10.1021/jm500791c. - DOI - PMC - PubMed
-
- Deo K.M., Ang D.L., McGhie B., Rajamanickam A., Dhiman A., Khoury A., Holland J., Bjelosevic A., Pages B., Gordon C., et al. Platinum Coordination Compounds with Potent Anticancer Activity. Coord. Chem. Rev. 2018;375:148–163. doi: 10.1016/j.ccr.2017.11.014. - DOI
-
- Jia C., Deacon G.B., Zhang Y., Gao C. Platinum(IV) Antitumor Complexes and Their Nano-Drug Delivery. Coord. Chem. Rev. 2021;429:213640. doi: 10.1016/j.ccr.2020.213640. - DOI
LinkOut - more resources
Full Text Sources

