Pharmacokinetics and Pharmacodynamics of Antibody-Drug Conjugates Administered via Subcutaneous and Intratumoral Routes
- PMID: 37111619
- PMCID: PMC10142912
- DOI: 10.3390/pharmaceutics15041132
Pharmacokinetics and Pharmacodynamics of Antibody-Drug Conjugates Administered via Subcutaneous and Intratumoral Routes
Abstract
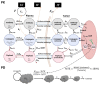
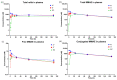
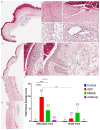
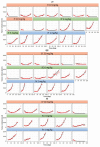
We hypothesize that different routes of administration may lead to altered pharmacokinetics/pharmacodynamics (PK/PD) behavior of antibody-drug conjugates (ADCs) and may help to improve their therapeutic index. To evaluate this hypothesis, here we performed PK/PD evaluation for an ADC administered via subcutaneous (SC) and intratumoral (IT) routes. Trastuzumab-vc-MMAE was used as the model ADC, and NCI-N87 tumor-bearing xenografts were used as the animal model. The PK of multiple ADC analytes in plasma and tumors, and the in vivo efficacy of ADC, after IV, SC, and IT administration were evaluated. A semi-mechanistic PK/PD model was developed to characterize all the PK/PD data simultaneously. In addition, local toxicity of SC-administered ADC was investigated in immunocompetent and immunodeficient mice. Intratumoral administration was found to significantly increase tumor exposure and anti-tumor activity of ADC. The PK/PD model suggested that the IT route may provide the same efficacy as the IV route at an increased dosing interval and reduced dose level. SC administration of ADC led to local toxicity and reduced efficacy, suggesting difficulty in switching from IV to SC route for some ADCs. As such, this manuscript provides unprecedented insight into the PK/PD behavior of ADCs after IT and SC administration and paves the way for clinical evaluation of these routes.
Keywords: antibody-drug conjugate (ADC); intratumoral administration; local toxicity; modeling and simulation (M&S); monomethyl auristatin E (MMAE); pharmacokinetics/pharmacodynamics (PK/PD); subcutaneous administration.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

