High-Throughput/High Content Imaging Screen Identifies Novel Small Molecule Inhibitors and Immunoproteasomes as Therapeutic Targets for Chordoma
- PMID: 37111759
- PMCID: PMC10145398
- DOI: 10.3390/pharmaceutics15041274
High-Throughput/High Content Imaging Screen Identifies Novel Small Molecule Inhibitors and Immunoproteasomes as Therapeutic Targets for Chordoma
Abstract
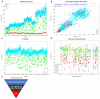
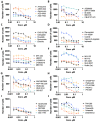
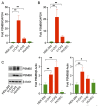
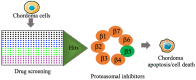
Chordomas account for approximately 1-4% of all malignant bone tumors and 20% of primary tumors of the spinal column. It is a rare disease, with an incidence estimated to be approximately 1 per 1,000,000 people. The underlying causative mechanism of chordoma is unknown, which makes it challenging to treat. Chordomas have been linked to the T-box transcription factor T (TBXT) gene located on chromosome 6. The TBXT gene encodes a protein transcription factor TBXT, or brachyury homolog. Currently, there is no approved targeted therapy for chordoma. Here, we performed a small molecule screening to identify small chemical molecules and therapeutic targets for treating chordoma. We screened 3730 unique compounds and selected 50 potential hits. The top three hits were Ribociclib, Ingenol-3-angelate, and Duvelisib. Among the top 10 hits, we found a novel class of small molecules, including proteasomal inhibitors, as promising molecules that reduce the proliferation of human chordoma cells. Furthermore, we discovered that proteasomal subunits PSMB5 and PSMB8 are increased in human chordoma cell lines U-CH1 and U-CH2, confirming that the proteasome may serve as a molecular target whose specific inhibition may lead to better therapeutic strategies for chordoma.
Keywords: cell proliferation; chordoma; high-throughput screening; proteasome; rare disease.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources

