Effect of Green Synthesized ZnO-NPs on Growth, Antioxidant System Response and Bioactive Compound Accumulation in Echinops macrochaetus, a Potential Medicinal Plant, and Assessment of Genome Size (2C DNA Content)
- PMID: 37111892
- PMCID: PMC10141689
- DOI: 10.3390/plants12081669
Effect of Green Synthesized ZnO-NPs on Growth, Antioxidant System Response and Bioactive Compound Accumulation in Echinops macrochaetus, a Potential Medicinal Plant, and Assessment of Genome Size (2C DNA Content)
Abstract
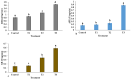
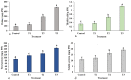
Echinops macrochaetus is a medicinal plant that can be used to cure various diseases. In the present study, plant-mediated zinc oxide nanoparticles (ZnO-NPs) were synthesized using an aqueous leaf extract of the medicinal plant Heliotropium bacciferum and characterized using various techniques. E. macrochaetus was collected from the wild and identified using the internal transcribed spacer sequence of nrDNA (ITS-nrDNA), which showed the closeness to its related genus in a phylogenetic tree. The effect of synthesized biogenic ZnO-NPs was studied on E. macrochaetus in a growth chamber for growth, bioactive compound enhancement and antioxidant system response. The irrigation of plants at a low concentration of ZnO-NPs (T1 = 10 mg/L) induced more growth in terms of biomass, chlorophyll content (273.11 µg/g FW) and carotenoid content (135.61 µg/g FW) than the control and other treatments (T2-20 mg/L and T3-40 mg/L). However, the application of a high concentration of ZnO-NPs (20 and 40 mg/L) increased the level of antioxidant enzymes (SOD, APX and GR), total crude and soluble protein, proline and TBARS contents. The accumulations of the compounds quercetin-3-β-D-glucoside, luteolin 7-rutinoside and p-coumaric acid were greater in the leaf compared to the shoot and root. A minor variation was observed in genome size in treated plants as compared to the control group. Overall, this study revealed the stimulatory effect of phytomediated ZnO-NPs, which act as bio-stimulants/nano-fertilizers as revealed by more biomass and the higher production of phytochemical compounds in different parts of the E. macrochaetus.
Keywords: antioxidant system response; genome size; medicinal plants; nano-fertilizers; polyphenolic compounds.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









References
-
- Funk V.A., Bayer R.J., Keeley S., Chan R., Watson L., Gemeinholzer B., Schilling E., Panero J.L., Baldwin B.G., Garcia-Jacas N. Everywhere but Antarctica: Using a supertree to understand the diversity and distribution of the Compositae. Biol. Skr. 2005;55:343–373.
-
- Arroo R., Jacobs J., Van Gestel J., Kenkel H., Jannink W., Croes A., Wullems G. Regulation of thiophene biosynthesis by sulphate in roots of marigolds. New Phytol. 1997;135:175–181. doi: 10.1046/j.1469-8137.1997.00637.x. - DOI
-
- Zamzami T.A., Abdallah H.M., Shehata I.A., Mohamed G.A., Alfaifi M.Y., Elbehairi S.E.I., Koshak A.E., Ibrahim S.R. Macrochaetosides A and B, new rare sesquiterpene glycosides from Echinops macrochaetus and their cytotoxic activity. Phytochem. Lett. 2019;30:88–92. doi: 10.1016/j.phytol.2019.01.025. - DOI
-
- Abegaz B.M. Polyacetylenic thiophenes and terpenoids from the roots of Echinops pappii. Phytochemistry. 1991;30:879–881. doi: 10.1016/0031-9422(91)85271-Z. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

