Chondrogenic Differentiation of Adipose-Derived Stromal Cells Induced by Decellularized Cartilage Matrix/Silk Fibroin Secondary Crosslinking Hydrogel Scaffolds with a Three-Dimensional Microstructure
- PMID: 37112015
- PMCID: PMC10144539
- DOI: 10.3390/polym15081868
Chondrogenic Differentiation of Adipose-Derived Stromal Cells Induced by Decellularized Cartilage Matrix/Silk Fibroin Secondary Crosslinking Hydrogel Scaffolds with a Three-Dimensional Microstructure
Abstract
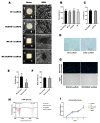
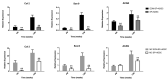
Finding an ideal scaffold is always an important issue in the field of cartilage tissue engineering. Both decellularized extracellular matrix and silk fibroin have been used as natural biomaterials for tissue regeneration. In this study, a secondary crosslinking method of γ irradiation and ethanol induction was used to prepare decellularized cartilage extracellular matrix and silk fibroin (dECM-SF) hydrogels with biological activity. Furthermore, the dECM-SF hydrogels were cast in custom-designed molds to produce a three-dimensional multi-channeled structure to improve internal connectivity. The adipose-derived stromal cells (ADSC) were seeded on the scaffolds, cultured in vitro for 2 weeks, and implanted in vivo for another 4 and 12 weeks. The double crosslinked dECM-SF hydrogels exhibited an excellent pore structure after lyophilization. The multi-channeled hydrogel scaffold presents higher water absorption ability, surface wettability, and no cytotoxicity. The addition of dECM and a channeled structure could promote chondrogenic differentiation of ADSC and engineered cartilage formation, confirmed by H&E, safranin O staining, type II collagen immunostaining, and qPCR assay. In conclusion, the hydrogel scaffold fabricated by the secondary crosslinking method has good plasticity and can be used as a scaffold for cartilage tissue engineering. The multi-channeled dECM-SF hydrogel scaffolds possess a chondrogenic induction activity that promotes engineered cartilage regeneration of ADSC in vivo.
Keywords: cartilage tissue engineering; decellularized cartilage extracellular matrix; multi-channeled; secondary crosslinking; silk fibroin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Crosslinker-free silk/decellularized extracellular matrix porous bioink for 3D bioprinting-based cartilage tissue engineering.Mater Sci Eng C Mater Biol Appl. 2021 Jan;118:111388. doi: 10.1016/j.msec.2020.111388. Epub 2020 Aug 22. Mater Sci Eng C Mater Biol Appl. 2021. PMID: 33254994
-
Combinatory approach for developing silk fibroin scaffolds for cartilage regeneration.Acta Biomater. 2018 May;72:167-181. doi: 10.1016/j.actbio.2018.03.047. Epub 2018 Apr 5. Acta Biomater. 2018. PMID: 29626700
-
Addition of Platelet-Rich Plasma to Silk Fibroin Hydrogel Bioprinting for Cartilage Regeneration.Tissue Eng Part A. 2020 Aug;26(15-16):886-895. doi: 10.1089/ten.TEA.2019.0304. Epub 2020 Mar 4. Tissue Eng Part A. 2020. PMID: 32031056
-
Cell-derived decellularized extracellular matrix scaffolds for articular cartilage repair.Int J Artif Organs. 2021 Apr;44(4):269-281. doi: 10.1177/0391398820953866. Epub 2020 Sep 18. Int J Artif Organs. 2021. PMID: 32945220 Review.
-
Decellularized extracellular matrix and silk fibroin-based hybrid biomaterials: A comprehensive review on fabrication techniques and tissue-specific applications.Int J Biol Macromol. 2023 Dec 31;253(Pt 8):127410. doi: 10.1016/j.ijbiomac.2023.127410. Epub 2023 Oct 14. Int J Biol Macromol. 2023. PMID: 37844823 Review.
Cited by
-
Preparation of silk fibroin-derived hydrogels and applications in skin regeneration.Health Sci Rep. 2024 Aug 12;7(8):e2295. doi: 10.1002/hsr2.2295. eCollection 2024 Aug. Health Sci Rep. 2024. PMID: 39139463 Free PMC article. Review.
-
Silk Fibroin Materials: Biomedical Applications and Perspectives.Bioengineering (Basel). 2024 Feb 9;11(2):167. doi: 10.3390/bioengineering11020167. Bioengineering (Basel). 2024. PMID: 38391652 Free PMC article. Review.
-
Hydrogel-Based 3D Bioprinting Technology for Articular Cartilage Regenerative Engineering.Gels. 2024 Jun 28;10(7):430. doi: 10.3390/gels10070430. Gels. 2024. PMID: 39057453 Free PMC article. Review.
-
Role of Ionizing Radiation Techniques in Polymeric Hydrogel Synthesis for Tissue Engineering Applications.Gels. 2025 Jan 8;11(1):47. doi: 10.3390/gels11010047. Gels. 2025. PMID: 39852018 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

