Functionalised Hybrid Collagen-Elastin for Acellular Cutaneous Substitute Applications
- PMID: 37112076
- PMCID: PMC10143773
- DOI: 10.3390/polym15081929
Functionalised Hybrid Collagen-Elastin for Acellular Cutaneous Substitute Applications
Abstract
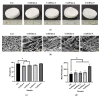
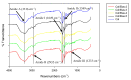
Wound contracture, which commonly happens after wound healing, may lead to physical distortion, including skin constriction. Therefore, the combination of collagen and elastin as the most abundant extracellular matrix (ECM) skin matrices may provide the best candidate biomaterials for cutaneous wound injury. This study aimed to develop a hybrid scaffold containing green natural resources (ovine tendon collagen type-I and poultry-based elastin) for skin tissue engineering. Briefly, freeze-drying was used to create the hybrid scaffolds, which were then crosslinked with 0.1% (w/v) genipin (GNP). Next, the physical characteristics (pore size, porosity, swelling ratio, biodegradability and mechanical strength) of the microstructure were assessed. Energy dispersive X-ray spectroscopy (EDX) and Fourier transform infrared (FTIR) spectrophotometry were used for the chemical analysis. The findings showed a uniform and interconnected porous structure with acceptable porosity (>60%) and high-water uptake capacity (>1200%), with pore sizes ranging between 127 ± 22 and 245 ± 35 µm. The biodegradation rate of the fabricated scaffold containing 5% elastin was lower (<0.043 mg/h) compared to the control scaffold (collagen only; 0.085 mg/h). Further analysis with EDX identified the main elements of the scaffold: it contained carbon (C) 59.06 ± 1.36-70.66 ± 2.89%, nitrogen (N) 6.02 ± 0.20-7.09 ± 0.69% and oxygen (O) 23.79 ± 0.65-32.93 ± 0.98%. FTIR analysis revealed that collagen and elastin remained in the scaffold and exhibited similar functional amides (amide A: 3316 cm-1, amide B: 2932 cm-1, amide I: 1649 cm-1, amide II: 1549 cm-1 and amide III: 1233 cm-1). The combination of elastin and collagen also produced a positive effect via increased Young's modulus values. No toxic effect was identified, and the hybrid scaffolds significantly supported human skin cell attachment and viability. In conclusion, the fabricated hybrid scaffolds demonstrated optimum physicochemical and mechanical properties and may potentially be used as an acellular skin substitute in wound management.
Keywords: acellular skin substitute; collagen; elastin; hybrid bioscaffold; tissue engineering.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Physicochemical Characterization of Bilayer Hybrid Nanocellulose-Collagen as a Potential Wound Dressing.Materials (Basel). 2020 Sep 30;13(19):4352. doi: 10.3390/ma13194352. Materials (Basel). 2020. PMID: 33007893 Free PMC article.
-
The Fabrication of Gelatin-Elastin-Nanocellulose Composite Bioscaffold as a Potential Acellular Skin Substitute.Polymers (Basel). 2023 Feb 3;15(3):779. doi: 10.3390/polym15030779. Polymers (Basel). 2023. PMID: 36772084 Free PMC article.
-
Characterization and Cytocompatibility of Collagen-Gelatin-Elastin (CollaGee) Acellular Skin Substitute towards Human Dermal Fibroblasts: In Vitro Assessment.Biomedicines. 2022 Jun 4;10(6):1327. doi: 10.3390/biomedicines10061327. Biomedicines. 2022. PMID: 35740348 Free PMC article.
-
Comprehensive Review of Hybrid Collagen and Silk Fibroin for Cutaneous Wound Healing.Materials (Basel). 2020 Jul 10;13(14):3097. doi: 10.3390/ma13143097. Materials (Basel). 2020. PMID: 32664418 Free PMC article. Review.
-
A Comprehensive Review on Collagen Type I Development of Biomaterials for Tissue Engineering: From Biosynthesis to Bioscaffold.Biomedicines. 2022 Sep 16;10(9):2307. doi: 10.3390/biomedicines10092307. Biomedicines. 2022. PMID: 36140407 Free PMC article. Review.
Cited by
-
Nanofiber Scaffolds as Drug Delivery Systems Promoting Wound Healing.Pharmaceutics. 2023 Jun 26;15(7):1829. doi: 10.3390/pharmaceutics15071829. Pharmaceutics. 2023. PMID: 37514015 Free PMC article. Review.
-
Efficacy and safety in synchronous core-needle biopsy and cryoablation for highly suspicious malignant pulmonary nodule.Cancer Imaging. 2025 Jun 21;25(1):78. doi: 10.1186/s40644-025-00901-0. Cancer Imaging. 2025. PMID: 40544289 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

