Electromagnetic Sensing Techniques for Monitoring Atopic Dermatitis-Current Practices and Possible Advancements: A Review
- PMID: 37112275
- PMCID: PMC10144024
- DOI: 10.3390/s23083935
Electromagnetic Sensing Techniques for Monitoring Atopic Dermatitis-Current Practices and Possible Advancements: A Review
Erratum in
-
Correction: Todorov et al. Electromagnetic Sensing Techniques for Monitoring Atopic Dermatitis-Current Practices and Possible Advancements: A Review. Sensors 2023, 23, 3935.Sensors (Basel). 2023 Dec 18;23(24):9888. doi: 10.3390/s23249888. Sensors (Basel). 2023. PMID: 38139759 Free PMC article.
Abstract
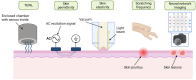
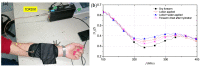
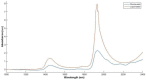
Atopic dermatitis (AD) is one of the most common skin disorders, affecting nearly one-fifth of children and adolescents worldwide, and currently, the only method of monitoring the condition is through an in-person visual examination by a clinician. This method of assessment poses an inherent risk of subjectivity and can be restrictive to patients who do not have access to or cannot visit hospitals. Advances in digital sensing technologies can serve as a foundation for the development of a new generation of e-health devices that provide accurate and empirical evaluation of the condition to patients worldwide. The goal of this review is to study the past, present, and future of AD monitoring. First, current medical practices such as biopsy, tape stripping and blood serum are discussed with their merits and demerits. Then, alternative digital methods of medical evaluation are highlighted with the focus on non-invasive monitoring using biomarkers of AD-TEWL, skin permittivity, elasticity, and pruritus. Finally, possible future technologies are showcased such as radio frequency reflectometry and optical spectroscopy along with a short discussion to provoke research into improving the current techniques and employing the new ones to develop an AD monitoring device, which could eventually facilitate medical diagnosis.
Keywords: atopic dermatitis; electromagnetic sensing; flexible wearable sensors; interdigitated capacitive sensor; near-infrared range spectroscopy; neural networks; non-invasive monitoring; radio frequency reflectometry; telemedical sensors; transepidermal water loss.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




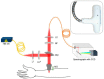
Similar articles
-
Use of Tape Strips to Detect Immune and Barrier Abnormalities in the Skin of Children With Early-Onset Atopic Dermatitis.JAMA Dermatol. 2019 Dec 1;155(12):1358-1370. doi: 10.1001/jamadermatol.2019.2983. JAMA Dermatol. 2019. PMID: 31596431 Free PMC article.
-
Sensitive skin is highly frequent in extrinsic atopic dermatitis and correlates with disease severity markers but not necessarily with skin barrier impairment.J Dermatol Sci. 2018 Jan;89(1):33-39. doi: 10.1016/j.jdermsci.2017.10.011. Epub 2017 Nov 2. J Dermatol Sci. 2018. PMID: 29122406
-
Comparison of skin hydration evaluation sites and correlations among skin hydration, transepidermal water loss, SCORAD index, Nottingham Eczema Severity Score, and quality of life in patients with atopic dermatitis.Am J Clin Dermatol. 2008;9(1):45-50. doi: 10.2165/00128071-200809010-00005. Am J Clin Dermatol. 2008. PMID: 18092843
-
Updates in atopic dermatitis for the primary care physician: A review of advances in the understanding and treatment of atopic dermatitis.Dis Mon. 2024 Apr;70(4):101687. doi: 10.1016/j.disamonth.2024.101687. Epub 2024 Jan 25. Dis Mon. 2024. PMID: 38278753 Review.
-
Dry skin in atopic dermatitis.Acta Derm Venereol Suppl (Stockh). 1992;177:9-13. Acta Derm Venereol Suppl (Stockh). 1992. PMID: 1466189 Review.
Cited by
-
Skin sensing and wearable technology as tools to measure atopic dermatitis severity.Skin Health Dis. 2024 Aug 15;4(5):e449. doi: 10.1002/ski2.449. eCollection 2024 Oct. Skin Health Dis. 2024. PMID: 39355726 Free PMC article. Review.
-
Correction: Todorov et al. Electromagnetic Sensing Techniques for Monitoring Atopic Dermatitis-Current Practices and Possible Advancements: A Review. Sensors 2023, 23, 3935.Sensors (Basel). 2023 Dec 18;23(24):9888. doi: 10.3390/s23249888. Sensors (Basel). 2023. PMID: 38139759 Free PMC article.
-
Bathing in Atopic Dermatitis in Pediatric Age: Why, How and When.Pediatr Rep. 2024 Jan 8;16(1):57-68. doi: 10.3390/pediatric16010006. Pediatr Rep. 2024. PMID: 38251315 Free PMC article. Review.
References
-
- Wagih M., Balocchi L., Benassi F., Carvalho N.B., Chiao J.-C., Correia R., Costanzo A., Cui Y., Georgiadou D., Gouveia C., et al. Microwave-Enabled Wearables: Underpinning Technologies, Integration Platforms, and Next-Generation Roadmap. IEEE J. Microw. 2023;3:193–226. doi: 10.1109/JMW.2022.3223254. - DOI
-
- De D., Kanwar A.J., Handa S. Comparative efficacy of Hanifin and Rajka’s criteria and the UK working party’s diagnostic criteria in diagnosis of atopic dermatitis in a hospital setting in North India. J. Eur. Acad. Dermatol. Venereol. 2006;20:853–859. doi: 10.1111/j.1468-3083.2006.01664.x. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

