The Difference between Rhizosphere and Endophytic Bacteria on the Safe Cultivation of Lettuce in Cr-Contaminated Farmland
- PMID: 37112598
- PMCID: PMC10146757
- DOI: 10.3390/toxics11040371
The Difference between Rhizosphere and Endophytic Bacteria on the Safe Cultivation of Lettuce in Cr-Contaminated Farmland
Abstract
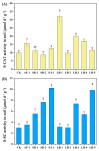
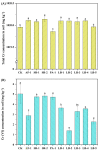
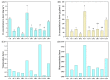
Chromium (Cr) is a major pollutant affecting the environment and human health and microbial remediation is considered to be the most promising technology for the restoration of the heavily metal-polluted soil. However, the difference between rhizosphere and endophytic bacteria on the potential of crop safety production in Cr-contaminated farmland is not clearly elucidated. Therefore, eight Cr-tolerant endophytic strains of three species: Serratia (SR-1~2), Lysinebacillus (LB-1~5) and Pseudomonas (PA-1) were isolated from rice and maize. Additionally, one Cr-tolerant strain of Alcaligenes faecalis (AF-1) was isolated from the rhizosphere of maize. A randomized group pot experiment with heavily Cr-contaminated (a total Cr concentration of 1020.18 mg kg-1) paddy clay soil was conducted and the effects of different bacteria on plant growth, absorption and accumulation of Cr in lettuce (Lactuca sativa var. Hort) were compared. The results show that: (i) the addition of SR-2, PA-1 and LB-5 could promote the accumulation of plant fresh weight by 10.3%, 13.5% and 14.2%, respectively; (ii) most of the bacteria could significantly increase the activities of rhizosphere soil catalase and sucrase, among which LB-1 promotes catalase activity by 224.60% and PA-1 increases sucrase activity by 247%; (iii) AF-1, SR-1, LB-1, SR-2, LB-2, LB-3, LB-4 and LB-5 strains could significantly decrease shoot the Cr concentration by 19.2-83.6%. The results reveal that Cr-tolerant bacteria have good potential to reduce shoot Cr concentration at the heavily contaminated soil and endophytic bacteria have the same or even better effects than rhizosphere bacteria; this suggests that bacteria in plants are more ecological friendly than bacteria in soil, thus aiming to safely produce crops in Cr-polluted farmland and alleviate Cr contamination from the food chain.
Keywords: Cr passivation; Cr-tolerant bacteria; Lactuca sativa L.; chromium; microbial remediation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
[Community Structure of Heavy Metal Immobilized Bacteria in the Lettuce (Lactuca sativa L.) Rhizosphere in Soil Polluted by Heavy Metals and Its Effects on Reducing Heavy Metal Accumulation in Lettuce].Huan Jing Ke Xue. 2019 Nov 8;40(11):5133-5141. doi: 10.13227/j.hjkx.201906055. Huan Jing Ke Xue. 2019. PMID: 31854583 Chinese.
-
Microbial communities in the rhizosphere of maize and cowpea respond differently to chromium contamination.Chemosphere. 2023 Feb;313:137417. doi: 10.1016/j.chemosphere.2022.137417. Epub 2022 Nov 29. Chemosphere. 2023. PMID: 36460149
-
The hyperaccumulator Sedum plumbizincicola harbors metal-resistant endophytic bacteria that improve its phytoextraction capacity in multi-metal contaminated soil.J Environ Manage. 2015 Jun 1;156:62-9. doi: 10.1016/j.jenvman.2015.03.024. Epub 2015 Mar 19. J Environ Manage. 2015. PMID: 25796039
-
Promises and potential of in situ nano-phytoremediation strategy to mycorrhizo-remediate heavy metal contaminated soils using non-food bioenergy crops (Vetiver zizinoides & Cannabis sativa).Int J Phytoremediation. 2020;22(9):900-915. doi: 10.1080/15226514.2020.1774504. Epub 2020 Jun 13. Int J Phytoremediation. 2020. PMID: 32538143 Review.
-
Phytoremediation as a management option for contaminated sediments in tidal marshes, flood control areas and dredged sediment landfill sites.Environ Sci Pollut Res Int. 2009 Nov;16(7):745-64. doi: 10.1007/s11356-009-0205-6. Epub 2009 Jun 16. Environ Sci Pollut Res Int. 2009. PMID: 19533193 Review.
Cited by
-
Microbial mediated remediation of heavy metals toxicity: mechanisms and future prospects.Front Plant Sci. 2024 Jul 19;15:1420408. doi: 10.3389/fpls.2024.1420408. eCollection 2024. Front Plant Sci. 2024. PMID: 39100088 Free PMC article. Review.
-
Exploring the potential of halotolerant bacteria from coastal regions to mitigate salinity stress in wheat: physiological, molecular, and biochemical insights.Front Plant Sci. 2023 Sep 22;14:1224731. doi: 10.3389/fpls.2023.1224731. eCollection 2023. Front Plant Sci. 2023. PMID: 37810397 Free PMC article.
References
-
- Katz S.A., Salem H. The Biological and Environmental Chemistry of Cr. VCH; New York, NY, USA: 1994. p. 214.
-
- Arisah F.M., Amir A.F., Ramli N., Ariffin H., Maeda T., Hassan M.A., Yusoff M.Z.M. Bacterial resistance against heavy metals in pseudomonas aeruginosa rw9 involving hexavalent Cr removal. Sustainability. 2021;13:9797. doi: 10.3390/su13179797. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

