Magnesium Supplementation Alleviates the Toxic Effects of Silica Nanoparticles on the Kidneys, Liver, and Adrenal Glands in Rats
- PMID: 37112608
- PMCID: PMC10141093
- DOI: 10.3390/toxics11040381
Magnesium Supplementation Alleviates the Toxic Effects of Silica Nanoparticles on the Kidneys, Liver, and Adrenal Glands in Rats
Abstract
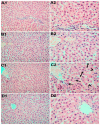
Concerns regarding the possible hazards to human health have been raised by the growing usage of silica nanoparticles (SiNPs) in a variety of applications, including industrial, agricultural, and medical applications. This in vivo subchronic study was conducted to assess the following: (1) the toxicity of orally administered SiNPs on the liver, kidneys, and adrenal glands; (2) the relationship between SiNPs exposure and oxidative stress; and (3) the role of magnesium in mitigating these toxic effects. A total of 24 Sprague Dawley male adult rats were divided equally into four groups, as follows: control group, magnesium (Mg) group (50 mg/kg/d), SiNPs group (100 mg/kg/d), and SiNPs+ Mg group. Rats were treated with SiNPs by oral gavage for 90 days. The liver transaminases, serum creatinine, and cortisol levels were evaluated. The tissue malondialdehyde (MDA) and reduced glutathione (GSH) levels were measured. Additionally, the weight of the organs and the histopathological changes were examined. Our results demonstrated that SiNPs exposure caused increased weight in the kidneys and adrenal glands. Exposure to SiNPs was also associated with significant alterations in liver transaminases, serum creatinine, cortisol, MDA, and GSH. Additionally, histopathological changes were significantly reported in the liver, kidneys, and adrenal glands of SiNPs-treated rats. Notably, when we compared the control group with the treated groups with SiNPs and Mg, the results revealed that magnesium could mitigate SiNPs-induced biochemical and histopathologic changes, confirming its effective role as an antioxidant that reduced the accumulation of SiNPs in tissues, and that it returns the levels of liver transaminases, serum creatinine, cortisol, MDA, and GSH to almost normal values.
Keywords: adrenal gland; kidney; liver; magnesium; oxidative stress; silica nanoparticles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Toxic Impacts of Amorphous Silica Nanoparticles on Liver and Kidney of Male Adult Rats: an In Vivo Study.Biol Trace Elem Res. 2021 Jul;199(7):2653-2662. doi: 10.1007/s12011-020-02386-3. Epub 2020 Sep 22. Biol Trace Elem Res. 2021. PMID: 32964349
-
Renal Fibrosis and Oxidative Stress Induced by Silica Nanoparticles in Male Rats and Its Molecular Mechanisms.Iran J Pharm Res. 2024 Mar 26;23(1):e143703. doi: 10.5812/ijpr-143703. eCollection 2024 Jan-Dec. Iran J Pharm Res. 2024. PMID: 38655071 Free PMC article.
-
Silica nanoparticles alleviate the immunosuppression, oxidative stress, biochemical, behavioral, and histopathological alterations induced by Aeromonas veronii infection in African catfish (Clarias gariepinus).Fish Physiol Biochem. 2024 Apr;50(2):767-783. doi: 10.1007/s10695-023-01274-6. Epub 2023 Dec 7. Fish Physiol Biochem. 2024. PMID: 38060081 Free PMC article.
-
Adverse effects and underlying mechanism of amorphous silica nanoparticles in liver.Chemosphere. 2023 Jan;311(Pt 1):136955. doi: 10.1016/j.chemosphere.2022.136955. Epub 2022 Oct 21. Chemosphere. 2023. PMID: 36280121 Review.
-
Radiolabeling Silica-Based Nanoparticles via Coordination Chemistry: Basic Principles, Strategies, and Applications.Acc Chem Res. 2018 Mar 20;51(3):778-788. doi: 10.1021/acs.accounts.7b00635. Epub 2018 Feb 28. Acc Chem Res. 2018. PMID: 29489335 Free PMC article. Review.
Cited by
-
Re-evaluation of silicon dioxide (E 551) as a food additive in foods for infants below 16 weeks of age and follow-up of its re-evaluation as a food additive for uses in foods for all population groups.EFSA J. 2024 Oct 17;22(10):e8880. doi: 10.2903/j.efsa.2024.8880. eCollection 2024 Oct. EFSA J. 2024. PMID: 39421729 Free PMC article.
References
-
- Bara N., Eshwarmoorthy M., Subaharan K., Kaul G. Mesoporous silica nanoparticle is comparatively safer than zinc oxide nanoparticle which can cause profound steroidogenic effects on pregnant mice and male offspring exposed in utero. Toxicol. Ind. Health. 2018;34:507–524. doi: 10.1177/0748233718757641. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

