Trans- and Multigenerational Effects of Isothiazolinone Biocide CMIT/MIT on Genotoxicity and Epigenotoxicity in Daphnia magna
- PMID: 37112615
- PMCID: PMC10140887
- DOI: 10.3390/toxics11040388
Trans- and Multigenerational Effects of Isothiazolinone Biocide CMIT/MIT on Genotoxicity and Epigenotoxicity in Daphnia magna
Abstract
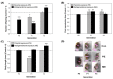
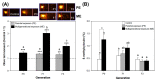
The mixture of 5-chloro-2-methylisothiazol-3(2H)-one and 2-methylisothiazol-3(2H)-one, CMIT/MIT, is an isothiazolinone biocide that is consistently detected in aquatic environments because of its broad-spectrum usage in industrial fields. Despite concerns about ecotoxicological risks and possible multigenerational exposure, toxicological information on CMIT/MIT is very limited to human health and within-generational toxicity. Furthermore, epigenetic markers altered by chemical exposure can be transmitted over generations, but the role of these changes in phenotypic responses and toxicity with respect to trans- and multigenerational effects is poorly understood. In this study, the toxicity of CMIT/MIT on Daphnia magna was evaluated by measuring various endpoints (mortality, reproduction, body size, swimming behavior, and proteomic expression), and its trans- and multigenerational effects were investigated over four consecutive generations. The genotoxicity and epigenotoxicity of CMIT/MIT were examined using a comet assay and global DNA methylation measurements. The results show deleterious effects on various endpoints and differences in response patterns according to different exposure histories. Parental effects were transgenerational or recovered after exposure termination, while multigenerational exposure led to acclimatory/defensive responses. Changes in DNA damage were closely associated with altered reproduction in daphnids, but their possible relationship with global DNA methylation was not found. Overall, this study provides ecotoxicological information on CMIT/MIT relative to multifaceted endpoints and aids in understanding multigenerational phenomena under CMIT/MIT exposure. It also emphasizes the consideration of exposure duration and multigenerational observations in evaluating ecotoxicity and the risk management of isothiazolinone biocides.
Keywords: aquatic toxicity; epigenotoxicity; genotoxicity; isothiazolinone; multigenerational effect; transgenerational effect.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Insights into the mechanisms of within-species variation in sensitivity to chemicals: A case study using daphnids exposed to CMIT/MIT biocide.Ecotoxicol Environ Saf. 2023 Jun 15;258:114967. doi: 10.1016/j.ecoenv.2023.114967. Epub 2023 May 9. Ecotoxicol Environ Saf. 2023. PMID: 37167738
-
Experimental determination of indoor air concentration of 5-chloro-2-methylisothiazol-3(2H)-one/ 2-methylisothiazol-3(2H)-one (CMIT/MIT) emitted by the use of humidifier disinfectant.Environ Anal Health Toxicol. 2020 Jun;35(2):e2020008. doi: 10.5620/eaht.e2020008. Epub 2020 Jun 30. Environ Anal Health Toxicol. 2020. PMID: 32600006 Free PMC article.
-
Risk Assessment of 5-Chloro-2-Methylisothiazol-3(2H)-One/2-Methylisothiazol-3(2H)-One (CMIT/MIT) Used as a Preservative in Cosmetics.Toxicol Res. 2019 Apr;35(2):103-117. doi: 10.5487/TR.2019.35.2.103. Epub 2019 Apr 15. Toxicol Res. 2019. PMID: 31015893 Free PMC article. Review.
-
Metabolite analysis of 14C-labeled chloromethylisothiazolinone/methylisothiazolinone for toxicological consideration of inhaled isothiazolinone biocides in lungs.Chemosphere. 2024 Aug;362:142666. doi: 10.1016/j.chemosphere.2024.142666. Epub 2024 Jun 21. Chemosphere. 2024. PMID: 38908450
-
Multigenerational tests on Daphnia spp.: a vision and new perspectives.Environ Pollut. 2023 Nov 15;337:122629. doi: 10.1016/j.envpol.2023.122629. Epub 2023 Sep 27. Environ Pollut. 2023. PMID: 37775025 Review.
Cited by
-
Metabolic Changes in Pseudomonas oleovorans Isolated from Contaminated Construction Material Exposed to Varied Biocide Treatments.Metabolites. 2024 Jun 10;14(6):326. doi: 10.3390/metabo14060326. Metabolites. 2024. PMID: 38921461 Free PMC article.
References
-
- Mardones L.E., Legnoverde M.S., Pereyra A.M., Basaldella E.I. Long-lasting isothiazolinone-based biocide obtained by encapsulation in micron-sized mesoporous matrices. Prog. Org. Coatings. 2018;119:155–163. doi: 10.1016/j.porgcoat.2018.02.023. - DOI
-
- Williams T.M. NACE-International Corrosion Conference Series. National Association of Corrosion Engineers International; Houston, Texas, USA: 2006. The mechanism of action of isothiazolone biocides.
Grants and funding
LinkOut - more resources
Full Text Sources

