Protective Effects of a Red Grape Juice Extract against Bisphenol A-Induced Toxicity in Human Umbilical Vein Endothelial Cells
- PMID: 37112618
- PMCID: PMC10145567
- DOI: 10.3390/toxics11040391
Protective Effects of a Red Grape Juice Extract against Bisphenol A-Induced Toxicity in Human Umbilical Vein Endothelial Cells
Abstract
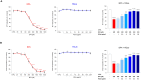
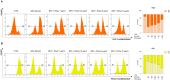
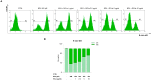
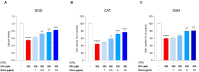
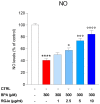
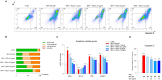
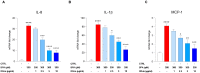
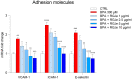
Human exposure to bisphenol A (BPA) occurs through the ingestion of contaminated food and water, thus leading to endothelial dysfunction, the first signal of atherosclerosis. Vitis vinifera L. (grape) juice is well known for its health-promoting properties, due to its numerous bioactive compounds among which are polyphenols. The aim of this study was to evaluate the protective effect of a red grape juice extract (RGJe) against the endothelial damage induced by BPA in human umbilical vein endothelial cells (HUVECs) as an in vitro model of endothelial dysfunction. Our results showed that RGJe treatment counteracted BPA-induced cell death and apoptosis in HUVECs, blocking caspase 3 and modulating p53, Bax, and Bcl-2. Moreover, RGJe demonstrated antioxidant properties in abiotic tests and in vitro, where it reduced BPA-induced reactive oxygen species as well as restored mitochondrial membrane potential, DNA integrity, and nitric oxide levels. Furthermore, RGJe reduced the increase of chemokines (IL-8, IL-1β, and MCP-1) and adhesion molecules (VCAM-1, ICAM-1, and E-selectin), caused by BPA exposure, involved in the primary phase of atheromatous plaque formation. Overall, our results suggest that RGJe prevents BPA-induced vascular damage modulating specific intracellular mechanisms, along with protecting cells, owing to its antioxidant capability.
Keywords: HUVECs; atherosclerosis; bisphenol A; cytotoxicity; endothelial dysfunction; oxidative stress; red grape juice extract.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Bisphenol A vascular toxicity: Protective effect of Vitis vinifera (grape) seed extract and resveratrol.Phytother Res. 2018 Dec;32(12):2396-2407. doi: 10.1002/ptr.6175. Epub 2018 Aug 16. Phytother Res. 2018. PMID: 30113097
-
Evaluation of green tea extract and epigallocatechin gallate effects on bisphenol A-induced vascular toxicity in isolated rat aorta and cytotoxicity in human umbilical vein endothelial cells.Phytother Res. 2021 Feb;35(2):996-1009. doi: 10.1002/ptr.6861. Epub 2020 Sep 7. Phytother Res. 2021. PMID: 32893422
-
Grape seed proanthocyanidin extract protects human umbilical vein endothelial cells from indoxyl sulfate-induced injury via ameliorating mitochondrial dysfunction.Ren Fail. 2016;38(1):100-8. doi: 10.3109/0886022X.2015.1104609. Epub 2015 Oct 29. Ren Fail. 2016. PMID: 26512753
-
Beta vulgaris rubra L. (Beetroot) Peel Methanol Extract Reduces Oxidative Stress and Stimulates Cell Proliferation via Increasing VEGF Expression in H2O2 Induced Oxidative Stressed Human Umbilical Vein Endothelial Cells.Genes (Basel). 2021 Aug 31;12(9):1380. doi: 10.3390/genes12091380. Genes (Basel). 2021. PMID: 34573361 Free PMC article.
-
Activation of Nrf2/HO-1 signaling: An important molecular mechanism of herbal medicine in the treatment of atherosclerosis via the protection of vascular endothelial cells from oxidative stress.J Adv Res. 2021 Jul 6;34:43-63. doi: 10.1016/j.jare.2021.06.023. eCollection 2021 Dec. J Adv Res. 2021. PMID: 35024180 Free PMC article. Review.
Cited by
-
Bisphenol A and Its Emergent Substitutes: State of the Art of the Impact of These Plasticizers on Oxidative Stress and Its Role in Vascular Dysfunction.Antioxidants (Basel). 2024 Nov 29;13(12):1468. doi: 10.3390/antiox13121468. Antioxidants (Basel). 2024. PMID: 39765797 Free PMC article. Review.
-
Toxicity of Metal Oxides, Dyes, and Dissolved Organic Matter in Water: Implications for the Environment and Human Health.Toxics. 2024 Jan 28;12(2):111. doi: 10.3390/toxics12020111. Toxics. 2024. PMID: 38393206 Free PMC article. Review.
-
The Inhibition of Mitogen-Activated Protein Kinases (MAPKs) and NF-κB Underlies the Neuroprotective Capacity of a Cinnamon/Curcumin/Turmeric Spice Blend in Aβ-Exposed THP-1 Cells.Molecules. 2023 Dec 5;28(24):7949. doi: 10.3390/molecules28247949. Molecules. 2023. PMID: 38138438 Free PMC article.
-
A comprehensive review of medicinal plants and their beneficial roles in alleviating bisphenol A-induced organ toxicity.Naunyn Schmiedebergs Arch Pharmacol. 2025 Jul;398(7):7801-7876. doi: 10.1007/s00210-025-03795-8. Epub 2025 Feb 11. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 39932506 Review.
-
G-Protein Coupled Receptor 1 Is Involved in Tetrachlorobisphenol A-Induced Inflammatory Response in Jurkat Cells.Toxics. 2024 Jul 2;12(7):485. doi: 10.3390/toxics12070485. Toxics. 2024. PMID: 39058137 Free PMC article.
References
-
- Montano L., Maugeri A., Volpe M.G., Micali S., Mirone V., Mantovani A., Navarra M., Piscopo M. Mediterranean Diet as a Shield against Male Infertility and Cancer Risk Induced by Environmental Pollutants: A Focus on Flavonoids. Int. J. Mol. Sci. 2022;23:1568. doi: 10.3390/ijms23031568. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

