Vaccinomics Approach for Multi-Epitope Vaccine Design against Group A Rotavirus Using VP4 and VP7 Proteins
- PMID: 37112638
- PMCID: PMC10144065
- DOI: 10.3390/vaccines11040726
Vaccinomics Approach for Multi-Epitope Vaccine Design against Group A Rotavirus Using VP4 and VP7 Proteins
Abstract
Rotavirus A is the most common cause of Acute Gastroenteritis globally among children <5 years of age. Due to a segmented genome, there is a high frequency of genetic reassortment and interspecies transmission which has resulted in the emergence of novel genotypes. There are concerns that monovalent (Rotarix: GlaxoSmithKline Biologicals, Rixensart, Belgium) and pentavalent (RotaTeq: MERCK & Co., Inc., Kenilworth, NJ, USA) vaccines may be less effective against non-vaccine strains, which clearly shows the demand for the design of a vaccine that is equally effective against all circulating genotypes. In the present study, a multivalent vaccine was designed from VP4 and VP7 proteins of RVA. Epitopes were screened for antigenicity, allergenicity, homology with humans and anti-inflammatory properties. The vaccine contains four B-cell, three CTL and three HTL epitopes joined via linkers and an N-terminal RGD motif adjuvant. The 3D structure was predicted and refined preceding its docking with integrin. Immune simulation displayed promising results both in Asia and worldwide. In the MD simulation, the RMSD value varied from 0.2 to 1.6 nm while the minimum integrin amino acid fluctuation (0.05-0.1 nm) was observed with its respective ligand. Codon optimization was performed with an adenovirus vector in a mammalian expression system. The population coverage analysis showed 99.0% and 98.47% in South Asia and worldwide, respectively. These computational findings show potential against all RVA genotypes; however, in-vitro/in-vivo screening is essential to devise a meticulous conclusion.
Keywords: acute gastroenteritis; in silico; multivalent; rotavirus; vaccinology; viruses.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


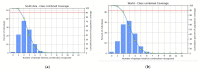
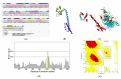


Similar articles
-
Genetic analyses reveal differences in the VP7 and VP4 antigenic epitopes between human rotaviruses circulating in Belgium and rotaviruses in Rotarix and RotaTeq.J Clin Microbiol. 2012 Mar;50(3):966-76. doi: 10.1128/JCM.05590-11. Epub 2011 Dec 21. J Clin Microbiol. 2012. PMID: 22189107 Free PMC article.
-
Sequence analysis of VP7 and VP4 genes of G1P[8] rotaviruses circulating among diarrhoeic children in Pune, India: a comparison with Rotarix and RotaTeq vaccine strains.Vaccine. 2014 Aug 11;32 Suppl 1:A75-83. doi: 10.1016/j.vaccine.2014.03.080. Vaccine. 2014. PMID: 25091685
-
Comparative characteristics of the VP7 and VP4 antigenic epitopes of the rotaviruses circulating in Russia (Nizhny Novgorod) and the Rotarix and RotaTeq vaccines.Arch Virol. 2015 Jul;160(7):1693-703. doi: 10.1007/s00705-015-2439-6. Epub 2015 May 7. Arch Virol. 2015. PMID: 25944143
-
Group A rotavirus universal mass vaccination: how and to what extent will selective pressure influence prevalence of rotavirus genotypes?Expert Rev Vaccines. 2012 Nov;11(11):1347-54. doi: 10.1586/erv.12.105. Expert Rev Vaccines. 2012. PMID: 23249234 Review.
-
Genetic diversity of G1P[8] rotavirus VP7 and VP8* antigens in Finland over a 20-year period: No evidence for selection pressure by universal mass vaccination with RotaTeq® vaccine.Infect Genet Evol. 2013 Oct;19:51-8. doi: 10.1016/j.meegid.2013.06.026. Epub 2013 Jul 4. Infect Genet Evol. 2013. PMID: 23831933 Review.
Cited by
-
Recombinant bivalent subunit vaccine combining truncated VP4 from P[7] and P[23] induces protective immunity against prevalent porcine rotaviruses.J Virol. 2024 May 14;98(5):e0021224. doi: 10.1128/jvi.00212-24. Epub 2024 Apr 9. J Virol. 2024. PMID: 38591886 Free PMC article.
-
Designing and development of efficient multi-epitope-based peptide vaccine candidate against emerging avian rotavirus strains: A vaccinomic approach.J Genet Eng Biotechnol. 2024 Sep;22(3):100398. doi: 10.1016/j.jgeb.2024.100398. Epub 2024 Jun 27. J Genet Eng Biotechnol. 2024. PMID: 39179326 Free PMC article.
-
Development of a novel bivalent vaccine candidate against hepatitis A virus and rotavirus using reverse vaccinology and immunoinformatics.J Virus Erad. 2025 Jan 23;11(1):100578. doi: 10.1016/j.jve.2024.100578. eCollection 2025 Mar. J Virus Erad. 2025. PMID: 40034561 Free PMC article.
-
Biotechnological Interventions for the Production of Subunit Vaccines Against Group A Rotavirus.Cell Biochem Funct. 2024 Dec;42(8):e70031. doi: 10.1002/cbf.70031. Cell Biochem Funct. 2024. PMID: 39707603 Free PMC article. Review.
-
Extended analyses of rotavirus C (RVC) G-types and P-types reveal new cut-off value for the G-types and reclassification of strains.J Virol. 2025 May 20;99(5):e0004925. doi: 10.1128/jvi.00049-25. Epub 2025 Apr 15. J Virol. 2025. PMID: 40231817 Free PMC article.
References
-
- Murray C.J., Aravkin A.Y., Zheng P., Abbafati C., Abbas K.M., Abbasi-Kangevari M., Abd-Allah F., Abdelalim A., Abdollahi M., Abdollahpour I., et al. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1223–1249. doi: 10.1016/S0140-6736(20)30752-2. - DOI - PMC - PubMed
-
- Yen C., Cortese M.M. In: 216—Rotaviruses, in Principles and Practice of Pediatric Infectious Diseases. 4th ed. Long S.S., editor. Elsevier; London, UK: 2012. pp. 1094–1097.
-
- Angel J., Franco M.A., Greenberg H.B. In: Rotaviruses, in Encyclopedia of Virology. 3rd ed. Mahy B.W.J., Van Regenmortel M.H.V., editors. Academic Press; Oxford, UK: 2008. pp. 507–513.
-
- Meštrović T. Rotavirus Structure and Classification. 2019. [(accessed on 13 January 2023)]. Available online: https://www.news-medical.net/health/Rotavirus-Structure-and-Classificati....
LinkOut - more resources
Full Text Sources

