Japanese Encephalitis Virus: An Update on the Potential Antivirals and Vaccines
- PMID: 37112654
- PMCID: PMC10146181
- DOI: 10.3390/vaccines11040742
Japanese Encephalitis Virus: An Update on the Potential Antivirals and Vaccines
Abstract
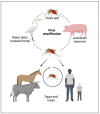
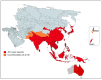
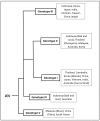
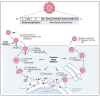
Japanese encephalitis virus (JEV) is the causal agent behind Japanese encephalitis (JE), a potentially severe brain infection that spreads through mosquito bites. JE is predominant over the Asia-Pacific Region and has the potential to spread globally with a higher rate of morbidity and mortality. Efforts have been made to identify and select various target molecules essential in JEV's progression, but until now, no licensed anti-JEV drug has been available. From a prophylactic point of view, a few licensed JE vaccines are available, but various factors, viz., the high cost and different side effects imposed by them, has narrowed their global use. With an average occurrence of >67,000 cases of JE annually, there is an urgent need to find a suitable antiviral drug to treat patients at the acute phase, as presently only supportive care is available to mitigate infection. This systematic review highlights the current status of efforts put in to develop antivirals against JE and the available vaccines, along with their effectiveness. It also summarizes epidemiology, structure, pathogenesis, and potential drug targets that can be explored to develop a new range of anti-JEV drugs to combat JEV infection globally.
Keywords: Japanese encephalitis (JE); Japanese encephalitis virus (JEV); antiviral; drug; vaccine.
Conflict of interest statement
All authors declare to have no conflict of interest.
Figures
Similar articles
-
Japanese encephalitis vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP).MMWR Recomm Rep. 2010 Mar 12;59(RR-1):1-27. MMWR Recomm Rep. 2010. PMID: 20224546
-
Natural products and derivatives as Japanese encephalitis virus antivirals.Pathog Dis. 2024 Feb 7;82:ftae022. doi: 10.1093/femspd/ftae022. Pathog Dis. 2024. PMID: 39317665 Free PMC article. Review.
-
Protection of swine by potent neutralizing anti-Japanese encephalitis virus monoclonal antibodies derived from vaccination.Antiviral Res. 2020 Feb;174:104675. doi: 10.1016/j.antiviral.2019.104675. Epub 2019 Dec 9. Antiviral Res. 2020. PMID: 31825852
-
Antiviral drug research for Japanese encephalitis: an updated review.Pharmacol Rep. 2022 Apr;74(2):273-296. doi: 10.1007/s43440-022-00355-2. Epub 2022 Feb 19. Pharmacol Rep. 2022. PMID: 35182390 Free PMC article. Review.
-
Axl Deficiency Promotes the Neuroinvasion of Japanese Encephalitis Virus by Enhancing IL-1α Production from Pyroptotic Macrophages.J Virol. 2020 Aug 17;94(17):e00602-20. doi: 10.1128/JVI.00602-20. Print 2020 Aug 17. J Virol. 2020. PMID: 32611752 Free PMC article.
Cited by
-
Molecular and Cellular Mechanisms Underlying Neurologic Manifestations of Mosquito-Borne Flavivirus Infections.Viruses. 2023 Oct 31;15(11):2200. doi: 10.3390/v15112200. Viruses. 2023. PMID: 38005878 Free PMC article. Review.
-
A chimeric vaccine derived from Australian genotype IV Japanese encephalitis virus protects mice from lethal challenge.NPJ Vaccines. 2024 Jul 31;9(1):134. doi: 10.1038/s41541-024-00903-2. NPJ Vaccines. 2024. PMID: 39085247 Free PMC article.
-
The alarming spread of Japanese encephalitis: A growing public health concern.Open Vet J. 2025 Apr;15(4):1505-1519. doi: 10.5455/OVJ.2025.v15.i4.1. Epub 2025 Apr 30. Open Vet J. 2025. PMID: 40453861 Free PMC article. Review.
-
In Children, N-Methyl-D-Aspartate Receptor Antibody Encephalitis Incidence Exceeds That of Japanese Encephalitis in Vietnam.Open Forum Infect Dis. 2024 Dec 6;11(12):ofae710. doi: 10.1093/ofid/ofae710. eCollection 2024 Dec. Open Forum Infect Dis. 2024. PMID: 39691294 Free PMC article.
-
Antiviral Efficacy of RNase H-Dependent Gapmer Antisense Oligonucleotides against Japanese Encephalitis Virus.Int J Mol Sci. 2023 Oct 2;24(19):14846. doi: 10.3390/ijms241914846. Int J Mol Sci. 2023. PMID: 37834294 Free PMC article.
References
-
- Burke D.S., Leake C.J. Japanese encephalitis. In: Monath T.P., editor. The Arboviruses: Epidemiology and Ecology. Volume 3. CRC Press; Boca Raton, FL, USA: 1988. pp. 63–92.
Publication types
LinkOut - more resources
Full Text Sources

