Cost-Effectiveness of Vaccination of Older Adults with an MF59®-Adjuvanted Quadrivalent Influenza Vaccine Compared to Standard-Dose and High-Dose Vaccines in Denmark, Norway, and Sweden
- PMID: 37112667
- PMCID: PMC10145635
- DOI: 10.3390/vaccines11040753
Cost-Effectiveness of Vaccination of Older Adults with an MF59®-Adjuvanted Quadrivalent Influenza Vaccine Compared to Standard-Dose and High-Dose Vaccines in Denmark, Norway, and Sweden
Abstract
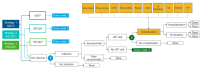
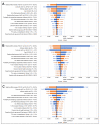
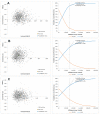
Individuals aged 65 years and above are at increased risk of complications and death from influenza compared with any other age group. Enhanced vaccines, as the MF59®-adjuvanted quadrivalent influenza vaccine (aQIV) and the high-dose quadrivalent influenza vaccine (HD-QIV), provide increased protection for older adults in comparison to the traditional standard-dose quadrivalent influenza vaccines (SD-QIV). This study aimed to assess the cost-effectiveness of aQIV compared to SD-QIV and HD-QIV in Denmark, Norway, and Sweden for adults aged ≥65 years. A static decision tree model was used to evaluate costs and outcomes of different vaccination strategies from healthcare payer and societal perspectives. This model projects that compared to SD-QIV, vaccination with aQIV could prevent a combined total of 18,772 symptomatic influenza infections, 925 hospitalizations, and 161 deaths in one influenza season across the three countries. From a healthcare payer perspective, the incremental costs per quality adjusted life year (QALY) gained with aQIV versus SD-QIV were EUR 10,170/QALY in Denmark, EUR 12,515/QALY in Norway, and EUR 9894/QALY in Sweden. The aQIV was cost saving compared with HD-QIV. This study found that introducing aQIV to the entire population aged ≥65 years may contribute to reducing the disease and economic burden associated with influenza in these countries.
Keywords: Nordic countries; adjuvanted influenza vaccine; cost-effectiveness; seasonal influenza.
Conflict of interest statement
J.J., W.M. and Y.S. are employed by IQVIA who was contracted by CSL Seqirus USA Inc. to conduct this study. T.B.-S., L.H.E., C.H.E., K.G.-I.M., A.N. and J.H received fees from IQVIA for their contributions to the research. J.M.-Q. and E.C. are employed by CSL Seqirus. J.M.-Q. is a shareholder of CSL Limited.
Figures
Similar articles
-
Real-World Evidence in Cost-Effectiveness Analysis of Enhanced Influenza Vaccines in Adults ≥ 65 Years of Age: Literature Review and Expert Opinion.Vaccines (Basel). 2023 Jun 11;11(6):1089. doi: 10.3390/vaccines11061089. Vaccines (Basel). 2023. PMID: 37376478 Free PMC article. Review.
-
The Cost-Effectiveness of Vaccination of Older Adults with an MF59-Adjuvanted Quadrivalent Influenza Vaccine Compared to Other Available Quadrivalent Vaccines in Germany.Vaccines (Basel). 2022 Aug 25;10(9):1386. doi: 10.3390/vaccines10091386. Vaccines (Basel). 2022. PMID: 36146464 Free PMC article.
-
An Economic Evaluation of the Adjuvanted Quadrivalent Influenza Vaccine Compared with Standard-Dose Quadrivalent Influenza Vaccine in the Spanish Older Adult Population.Vaccines (Basel). 2022 Aug 20;10(8):1360. doi: 10.3390/vaccines10081360. Vaccines (Basel). 2022. PMID: 36016247 Free PMC article.
-
Cost-Effectiveness of Adjuvanted Influenza Vaccine Compared with Standard and High-Dose Influenza Vaccines for Persons Aged ≥50 Years in Spain.Vaccines (Basel). 2025 Mar 19;13(3):323. doi: 10.3390/vaccines13030323. Vaccines (Basel). 2025. PMID: 40266221 Free PMC article.
-
Effectiveness of the MF59-adjuvanted trivalent or quadrivalent seasonal influenza vaccine among adults 65 years of age or older, a systematic review and meta-analysis.Influenza Other Respir Viruses. 2021 Nov;15(6):813-823. doi: 10.1111/irv.12871. Epub 2021 Jun 3. Influenza Other Respir Viruses. 2021. PMID: 34081398 Free PMC article.
Cited by
-
The role of vaccination in COPD: influenza, SARS-CoV-2, pneumococcus, pertussis, RSV and varicella zoster virus.Eur Respir Rev. 2023 Sep 6;32(169):230034. doi: 10.1183/16000617.0034-2023. Print 2023 Sep 30. Eur Respir Rev. 2023. PMID: 37673427 Free PMC article.
-
Real-World Evidence in Cost-Effectiveness Analysis of Enhanced Influenza Vaccines in Adults ≥ 65 Years of Age: Literature Review and Expert Opinion.Vaccines (Basel). 2023 Jun 11;11(6):1089. doi: 10.3390/vaccines11061089. Vaccines (Basel). 2023. PMID: 37376478 Free PMC article. Review.
-
Influential drivers of the cost-effectiveness of respiratory syncytial virus vaccination in European older adults: a multi-country analysis.BMC Med. 2025 Mar 24;23(1):170. doi: 10.1186/s12916-025-03970-x. BMC Med. 2025. PMID: 40128710 Free PMC article.
-
Health equity e sostenibilità nella vaccinazione antinfluenzale degli adulti over 65 in Italia.J Prev Med Hyg. 2024 Oct 23;65(3 Suppl 1):E1-E8. doi: 10.15167/2421-4248/jpmh2024.65.3s1. eCollection 2024 Sep. J Prev Med Hyg. 2024. PMID: 39539318 Free PMC article. Italian. No abstract available.
-
Cost-Effectiveness of Adjuvanted Quadrivalent Influenza Vaccine for Adults over 65 in France.Vaccines (Basel). 2024 May 24;12(6):574. doi: 10.3390/vaccines12060574. Vaccines (Basel). 2024. PMID: 38932304 Free PMC article.
References
-
- World Health Organization (WHO) Influenza (Seasonal) [(accessed on 31 October 2022)]; Available online: https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal)
-
- World Health Organization (WHO) Influenza. [(accessed on 31 October 2022)]; Available online: https://www.who.int/teams/health-product-policy-and-standards/standards-....
-
- European Centre for Disease Prevention and Control Factsheet about Seasonal Influenza. [(accessed on 28 October 2022)]. Available online: https://www.ecdc.europa.eu/en/seasonal-influenza/facts/factsheet.
-
- Hak E., Wei F., Nordin J., Mullooly J., Poblete S., Nichol K.L. Development and validation of a clinical prediction rule for hospitalization due to pneumonia or influenza or death during influenza epidemics among community-dwelling elderly persons. J. Infect. Dis. 2004;189:450–458. doi: 10.1086/381165. - DOI - PubMed
LinkOut - more resources
Full Text Sources