Intradermal Immunization of Soluble Influenza HA Derived from a Lethal Virus Induces High Magnitude and Breadth of Antibody Responses and Provides Complete Protection In Vivo
- PMID: 37112692
- PMCID: PMC10141624
- DOI: 10.3390/vaccines11040780
Intradermal Immunization of Soluble Influenza HA Derived from a Lethal Virus Induces High Magnitude and Breadth of Antibody Responses and Provides Complete Protection In Vivo
Abstract
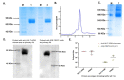
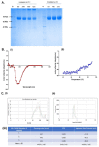
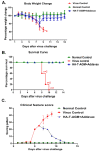
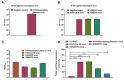
Immunogens mimicking the native-like structure of surface-exposed viral antigens are considered promising vaccine candidates. Influenza viruses are important zoonotic respiratory viruses with high pandemic potential. Recombinant soluble hemagglutinin (HA) glycoprotein-based protein subunit vaccines against Influenza have been shown to induce protective efficacy when administered intramuscularly. Here, we have expressed a recombinant soluble trimeric HA protein in Expi 293F cells and purified the protein derived from the Inf A/Guangdong-Maonan/ SWL1536/2019 virus which was found to be highly virulent in the mouse. The trimeric HA protein was found to be in the oligomeric state, highly stable, and the efficacy study in the BALB/c mouse challenge model through intradermal immunization with the prime-boost regimen conferred complete protection against a high lethal dose of homologous and mouse-adapted InfA/PR8 virus challenge. Furthermore, the immunogen induced high hemagglutinin inhibition (HI) titers and showed cross-protection against other Inf A and Inf B subtypes. The results are promising and warrant trimeric HA as a suitable vaccine candidate.
Keywords: hemagglutinin; influenza; intradermal route; vaccine; virus.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


Similar articles
-
Immunization with baculovirus displayed H6 hemagglutinin vaccine protects mice against lethal H6 influenza virus challenge.Antiviral Res. 2014 Sep;109:42-53. doi: 10.1016/j.antiviral.2014.06.002. Epub 2014 Jun 25. Antiviral Res. 2014. PMID: 24973759
-
Recombinant parainfluenza virus 5 expressing clade 2.3.4.4b H5 hemagglutinin protein confers broad protection against H5Ny influenza viruses.J Virol. 2024 Mar 19;98(3):e0112923. doi: 10.1128/jvi.01129-23. Epub 2024 Feb 2. J Virol. 2024. PMID: 38305155 Free PMC article.
-
A Replication-Defective Influenza Virus Harboring H5 and H7 Hemagglutinins Provides Protection against H5N1 and H7N9 Infection in Mice.J Virol. 2021 Jan 13;95(3):e02154-20. doi: 10.1128/JVI.02154-20. Print 2021 Jan 13. J Virol. 2021. PMID: 33177192 Free PMC article.
-
Vaccination with Recombinant Parainfluenza Virus 5 Expressing Neuraminidase Protects against Homologous and Heterologous Influenza Virus Challenge.J Virol. 2017 Nov 14;91(23):e01579-17. doi: 10.1128/JVI.01579-17. Print 2017 Dec 1. J Virol. 2017. PMID: 28931689 Free PMC article.
-
Synthetic Toll-like receptor 4 (TLR4) and TLR7 ligands as influenza virus vaccine adjuvants induce rapid, sustained, and broadly protective responses.J Virol. 2015 Mar;89(6):3221-35. doi: 10.1128/JVI.03337-14. Epub 2015 Jan 7. J Virol. 2015. PMID: 25568203 Free PMC article.
Cited by
-
Ferritin-Based HA DNA Vaccine Outperforms Conventional Designs in Inducing Protective Immunity Against Seasonal Influenza.Vaccines (Basel). 2025 Jul 10;13(7):745. doi: 10.3390/vaccines13070745. Vaccines (Basel). 2025. PMID: 40733722 Free PMC article.
-
Immunogenic and Protective Properties of Recombinant Hemagglutinin of Influenza A (H5N8) Virus.Vaccines (Basel). 2024 Jan 29;12(2):143. doi: 10.3390/vaccines12020143. Vaccines (Basel). 2024. PMID: 38400127 Free PMC article.
-
Advances in Human Pathogen Control-A 21st Century Challenge.Vaccines (Basel). 2023 Sep 2;11(9):1449. doi: 10.3390/vaccines11091449. Vaccines (Basel). 2023. PMID: 37766126 Free PMC article.
References
-
- Havers F.P., Campbell A.J.P. Nelson Textbook of Pediatrics. Elsevier; Amsterdam, The Netherlands: 2020. Influenza Viruses; pp. 1727–1732.e1. Chapter 285.
Grants and funding
LinkOut - more resources
Full Text Sources

