Immunogenicity Differences of the ChAdOx1 nCoV-19 Vaccine According to Pre-Existing Adenovirus Immunity
- PMID: 37112696
- PMCID: PMC10145356
- DOI: 10.3390/vaccines11040784
Immunogenicity Differences of the ChAdOx1 nCoV-19 Vaccine According to Pre-Existing Adenovirus Immunity
Abstract
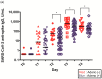
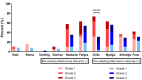
This study investigated the immunogenicity of, and reactogenicity to, the ChAdOx1 nCoV-19 vaccine according to pre-existing adenovirus immunity. Individuals scheduled for COVID-19 vaccination were prospectively enrolled in a tertiary hospital with 2400 beds from March 2020 onwards. Pre-existing adenovirus immunity data was obtained before ChAdOx1 nCoV-19 vaccination. A total of 68 adult patients administered two doses of the ChAdOx1 nCoV-19 vaccine were enrolled. Pre-existing adenovirus immunity was identified in 49 patients (72.1%), but not in the remaining 19 patients (27.9%). The geometric mean titer of S-specific IgG antibodies was statistically higher in individuals without pre-existing adenovirus immunity at several time points: before the second ChAdOx1 nCoV-19 dose (56.4 (36.6-125.0) vs. 51.0 (17.9-122.3), p = 0.024), 2-3 weeks after the second ChAdOx1 nCoV-19 dose (629.5 (451.5-926.5) vs. 555.0 (287.3-926.0), p = 0.049), and 3 months after the second ChAdOx1 nCoV-19 dose (274.5 (160.5-655.3) vs. 176.0 (94.3-255.3), p = 0.033). In the absence of pre-existing adenovirus immunity, systemic events were observed with higher frequency, especially chills (73.7% vs. 31.9%, p = 0.002). In conclusion, individuals without pre-existing adenovirus immunity showed a higher immune response to ChAdOx1 nCoV-19 vaccination and a higher frequency of reactogenicity to ChAdOx1 nCoV-19 vaccination was observed.
Keywords: ChAdOx1 nCoV-19; adenoviridae; adenovirus vaccines; immunogenicity; vaccine.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


Similar articles
-
Tolerability and immunogenicity of an intranasally-administered adenovirus-vectored COVID-19 vaccine: An open-label partially-randomised ascending dose phase I trial.EBioMedicine. 2022 Nov;85:104298. doi: 10.1016/j.ebiom.2022.104298. Epub 2022 Oct 10. EBioMedicine. 2022. PMID: 36229342 Free PMC article. Clinical Trial.
-
Safety, reactogenicity, and immunogenicity of homologous and heterologous prime-boost immunisation with ChAdOx1 nCoV-19 and BNT162b2: a prospective cohort study.Lancet Respir Med. 2021 Nov;9(11):1255-1265. doi: 10.1016/S2213-2600(21)00357-X. Epub 2021 Aug 13. Lancet Respir Med. 2021. PMID: 34391547 Free PMC article.
-
Safety and immunogenicity of the ChAdOx1 nCoV-19 (AZD1222) vaccine in children aged 6-17 years: a preliminary report of COV006, a phase 2 single-blind, randomised, controlled trial.Lancet. 2022 Jun 11;399(10342):2212-2225. doi: 10.1016/S0140-6736(22)00770-X. Lancet. 2022. PMID: 35691324 Free PMC article. Clinical Trial.
-
COVID-19 vaccine type-dependent differences in immunogenicity and inflammatory response: BNT162b2 and ChAdOx1 nCoV-19.Front Immunol. 2022 Sep 2;13:975363. doi: 10.3389/fimmu.2022.975363. eCollection 2022. Front Immunol. 2022. PMID: 36119092 Free PMC article.
-
ChAdOx1-S vaccine for prevention of COVID-19.Aust Prescr. 2021 Apr;44(2):59-61. doi: 10.18773/austprescr.2021.012. Epub 2021 Mar 10. Aust Prescr. 2021. PMID: 33911335 Free PMC article. Review. No abstract available.
Cited by
-
A novel orf virus vector-based COVID-19 booster vaccine shows cross-neutralizing activity in the absence of anti-vector neutralizing immunity.Hum Vaccin Immunother. 2024 Dec 31;20(1):2410574. doi: 10.1080/21645515.2024.2410574. Epub 2024 Oct 14. Hum Vaccin Immunother. 2024. PMID: 39397784 Free PMC article. Clinical Trial.
-
The Immune System-A Double-Edged Sword for Adenovirus-Based Therapies.Viruses. 2024 Jun 17;16(6):973. doi: 10.3390/v16060973. Viruses. 2024. PMID: 38932265 Free PMC article. Review.
-
Vaccine delivery systems and administration routes: Advanced biotechnological techniques to improve the immunization efficacy.Vaccine X. 2024 May 24;19:100500. doi: 10.1016/j.jvacx.2024.100500. eCollection 2024 Aug. Vaccine X. 2024. PMID: 38873639 Free PMC article. Review.
References
-
- Buchbinder S.P., Mehrotra D.V., Duerr A., Fitzgerald D.W., Mogg R., Li D., Gilbert P.B., Lama J.R., Marmor M., Del Rio C., et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): A double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372:1881–1893. doi: 10.1016/S0140-6736(08)61591-3. - DOI - PMC - PubMed
-
- Harro C.D., Robertson M.N., Lally M.A., O’Neill L.D., Edupuganti S., Goepfert P.A., Mulligan M.J., Priddy F.H., Dubey S.A., Kierstead L.S., et al. Safety and immunogenicity of adenovirus-vectored near-consensus HIV type 1 clade B gag vaccines in healthy adults. AIDS Res. Hum. Retrovir. 2009;25:103–114. doi: 10.1089/aid.2008.0212. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

