Short- and Long-Term Humoral and Cellular Immune Responses to SARS-CoV-2 Vaccination in Patients with Multiple Sclerosis Treated with Disease-Modifying Therapies
- PMID: 37112698
- PMCID: PMC10145338
- DOI: 10.3390/vaccines11040786
Short- and Long-Term Humoral and Cellular Immune Responses to SARS-CoV-2 Vaccination in Patients with Multiple Sclerosis Treated with Disease-Modifying Therapies
Abstract
Background: This study aimed to evaluate short- and long-term humoral and T-cell-specific immune responses to SARS-CoV-2 vaccines in patients with multiple sclerosis (MS) treated with different disease-modifying therapies (DMTs).
Methods: Single-center observational longitudinal study including 102 patients with MS who consecutively received vaccination against SARS-CoV-2. Serum samples were collected at baseline and after receiving the second dose of the vaccine. Specific Th1 responses following in vitro stimulation with spike and nucleocapsid peptides were analyzed by quantifying levels of IFN-γ. Serum IgG-type antibodies against the spike region of SARS-CoV-2 were studied by chemiluminescent microparticle immunoassay.
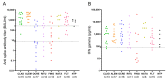
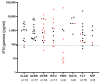
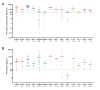
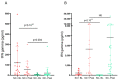
Results: Patients undergoing fingolimod and anti-CD20 therapies had a markedly lower humoral response than those treated with other DMTs and untreated patients. Robust antigen-specific T-cell responses were detected in all patients except those treated with fingolimod, who had lower IFN-γ levels than those treated with other DMTs (25.8 pg/mL vs. 868.7 pg/mL, p = 0.011). At mid-term follow-up, a decrease in vaccine-induced anti-SARS-CoV-2 IgG antibodies was observed in all subgroups of patients receiving DMTs, although most patients receiving induction DMTs or natalizumab and non-treated patients remained protected. Cellular immunity was maintained above protective levels in all DMT subgroups except the fingolimod subgroup.
Conclusions: SARS-CoV-2 vaccines induce robust and long-lasting humoral and cell-mediated specific immune responses in most patients with MS.
Keywords: COVID19; SARS-CoV-2 vaccination; disease-modifying therapies; immune response; multiple sclerosis.
Conflict of interest statement
S.S.M. received research grants, travel support or honoraria for speaking engagements from Almirall, Biogen, Bristol Myers Squibb, Janssen, Merck, Mylan, Novartis, Roche, Sanofi-Genzyme, and Teva. E.M. received research grants, travel support or honoraria for speaking engagements from Almirall, Biogen, Janssen, Merck, Novartis, Roche, and Sanofi-Genzyme. L.C.-F. received speaker fees and travel support from, and/or served on advisory boards by, Almirall, Bayer, Biogen, Biopas, Bristol Myers Squibb, Celgene, Ipsen, Janssen, Merck, Novartis, Roche, Sanofi and Teva. L.V. received research grants, travel support or honoraria for speaking engagements from Biogen, Bristol Myers Squibb, Merck, Novartis, Roche and Sanofi-Genzyme. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Tur C., Dubessy A.L., Otero-Romero S., Amato M.P., Derfuss T., Di Pauli F., Iacobaeus E., Mycko M., Abboud H., Achiron A., et al. The risk of infections for multiple sclerosis and neuromyelitis optica spectrum disorder disease-modifying treatments: Eighth European Committee for Treatment and Research in Multiple Sclerosis Focused Workshop Review. April 2021. Mult. Scler. 2022;28:1424–1456. doi: 10.1177/13524585211069068. - DOI - PubMed
-
- Farez M.F., Correale J., Armstrong M.J., Rae-Grant A., Gloss D., Donley D., Holler-Managan Y., Kachuck N.J., Jeffery D., Beilman M., et al. Practice guideline update summary: Vaccine-preventable infections and immunization in multiple sclerosis: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2019;93:584–594. doi: 10.1212/WNL.0000000000008157. - DOI - PubMed
-
- Moiola L., Barcella V., Benatti S., Capobianco M., Capra R., Cinque P., Comi G., Fasolo M.M., Franzetti F., Galli M., et al. The risk of infection in patients with multiple sclerosis treated with disease-modifying therapies: A Delphi consensus statement. Mult. Scler. 2021;27:331–346. doi: 10.1177/1352458520952311. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

