Immunogenicity of a Third Dose of BNT162b2 Vaccine among Lung Transplant Recipients-A Prospective Cohort Study
- PMID: 37112711
- PMCID: PMC10141618
- DOI: 10.3390/vaccines11040799
Immunogenicity of a Third Dose of BNT162b2 Vaccine among Lung Transplant Recipients-A Prospective Cohort Study
Abstract
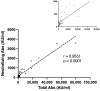
Two doses of mRNA SARS-CoV-2 vaccines elicit an attenuated humoral immune response among immunocompromised patients. Our study aimed to assess the immunogenicity of a third dose of the BNT162b2 vaccine among lung transplant recipients (LTRs). We prospectively evaluated the humoral response by measuring anti-spike SARS-CoV-2 and neutralizing antibodies in 139 vaccinated LTRs ~4-6 weeks following the third vaccine dose. The t-cell response was evaluated by IFNγ assay. The primary outcome was the seropositivity rate following the third vaccine dose. Secondary outcomes included: positive neutralizing antibody and cellular immune response rate, adverse events, and COVID-19 infections. Results were compared to a control group of 41 healthcare workers. Among LTRs, 42.4% had a seropositive antibody titer, and 17.2% had a positive t-cell response. Seropositivity was associated with younger age (t = 3.736, p < 0.001), higher GFR (t = 2.355, p = 0.011), and longer duration from transplantation (t = -1.992, p = 0.024). Antibody titer positively correlated with neutralizing antibodies (r = 0.955, p < 0.001). The current study may suggest the enhancement of immunogenicity by using booster doses. Since monoclonal antibodies have limited effectiveness against prevalent sub-variants and LTRs are prone to severe COVID-19 morbidity, vaccination remains crucial for this vulnerable population.
Keywords: BNT162b2 COVID-19 vaccine; immunogenicity; lung transplantation; third dose.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Third dose of BNT162b2 improves immune response in liver transplant recipients to ancestral strain but not Omicron BA.1 and XBB.Front Immunol. 2023 Jul 3;14:1206016. doi: 10.3389/fimmu.2023.1206016. eCollection 2023. Front Immunol. 2023. PMID: 37465685 Free PMC article.
-
Lung Transplant Recipients Immunogenicity after Heterologous ChAdOx1 nCoV-19-BNT162b2 mRNA Vaccination.Viruses. 2022 Jul 2;14(7):1470. doi: 10.3390/v14071470. Viruses. 2022. PMID: 35891450 Free PMC article.
-
Comparison of Antibody Response Elicited by ChAdOx1 and BNT162b2 COVID-19 Vaccine.J Korean Med Sci. 2021 Nov 29;36(46):e311. doi: 10.3346/jkms.2021.36.e311. J Korean Med Sci. 2021. PMID: 34845875 Free PMC article.
-
Comparison of SARS-CoV-2 Antibody Response 4 Weeks After Homologous vs Heterologous Third Vaccine Dose in Kidney Transplant Recipients: A Randomized Clinical Trial.JAMA Intern Med. 2022 Feb 1;182(2):165-171. doi: 10.1001/jamainternmed.2021.7372. JAMA Intern Med. 2022. PMID: 34928302 Free PMC article. Clinical Trial.
-
Seroconversion following a booster dose of COVID-19 vaccine in liver transplant recipients: a systematic review and meta-analysis.Pol Arch Intern Med. 2023 Sep 29;133(9):16455. doi: 10.20452/pamw.16455. Epub 2023 Mar 6. Pol Arch Intern Med. 2023. PMID: 36876925
Cited by
-
COVID-19 in Lung Transplant Recipients: A Report on 10 Recent Cases.Viruses. 2024 Apr 29;16(5):709. doi: 10.3390/v16050709. Viruses. 2024. PMID: 38793590 Free PMC article.
References
-
- WHO Coronavirus (COVID-19) Dashboard|WHO Coronavirus (COVID-19) Dashboard with Vaccination Data. [(accessed on 9 January 2023)]. Available online: https://covid19.who.int/
-
- Jamison D.A., Anand Narayanan S., Trovão N.S., Guarnieri J.W., Topper M.J., Moraes-Vieira P.M., Zaksas V., Singh K.K., Wurtele E.S., Beheshti A. A comprehensive SARS-CoV-2 and COVID-19 review, Part 1: Intracellular overdrive for SARS-CoV-2 infection. Eur. J. Hum. Genet. 2022;30:889–898. doi: 10.1038/s41431-022-01108-8. - DOI - PMC - PubMed
-
- FDA Announces Evusheld Is Not Currently Authorized for Emergency Use in the U.S.|FDA. [(accessed on 30 January 2023)]; Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-announces-evu....
LinkOut - more resources
Full Text Sources
Miscellaneous

