Development of MVA-d34 Tetravalent Dengue Vaccine: Design and Immunogenicity
- PMID: 37112743
- PMCID: PMC10142911
- DOI: 10.3390/vaccines11040831
Development of MVA-d34 Tetravalent Dengue Vaccine: Design and Immunogenicity
Abstract
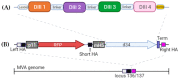
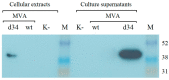
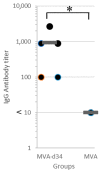
Dengue fever, an infectious disease that affects more than 100 million people every year, is a global health problem. Vaccination may be the most effective prevention strategy for the disease. However, the development of vaccines against dengue fever is complicated by the high risk of developing an antibody-dependent increase in infection. This article describes the development of an MVA-d34 vaccine against the dengue virus based on a safe and effective MVA viral vector. The DIII domains of the envelope protein (E) of the dengue virus are used as vaccine antigens, as antibodies against these domains do not cause an enhancement of infection. The use of the DIII domains of each of the four dengue virus serotypes made it possible to generate a humoral response against all four dengue virus serotypes in immunized mice. We also showed that the sera of vaccinated mice present virus-neutralizing activity against dengue serotype 2. Thus, the developed MVA-d34 vaccine is a promising candidate vaccine against dengue fever.
Keywords: MVA; dengue; vaccine.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
An Envelope-Modified Tetravalent Dengue Virus-Like-Particle Vaccine Has Implications for Flavivirus Vaccine Design.J Virol. 2017 Nov 14;91(23):e01181-17. doi: 10.1128/JVI.01181-17. Print 2017 Dec 1. J Virol. 2017. PMID: 28956764 Free PMC article.
-
Universal Dengue Vaccine Elicits Neutralizing Antibodies against Strains from All Four Dengue Virus Serotypes.J Virol. 2021 Jan 28;95(4):e00658-20. doi: 10.1128/JVI.00658-20. Print 2021 Jan 28. J Virol. 2021. PMID: 33208445 Free PMC article.
-
Induction of protective antibodies against dengue virus by tetravalent DNA immunization of mice with domain III of the envelope protein.Vaccine. 2005 May 16;23(26):3469-76. doi: 10.1016/j.vaccine.2004.12.028. Vaccine. 2005. PMID: 15837370
-
Dengue viruses and promising envelope protein domain III-based vaccines.Appl Microbiol Biotechnol. 2018 Apr;102(7):2977-2996. doi: 10.1007/s00253-018-8822-y. Epub 2018 Feb 22. Appl Microbiol Biotechnol. 2018. PMID: 29470620 Review.
-
Recent progress in dengue vaccine research and development.Curr Opin Mol Ther. 2010 Feb;12(1):31-8. Curr Opin Mol Ther. 2010. PMID: 20140814 Review.
Cited by
-
An effective pan-serotype dengue vaccine and enhanced control strategies could help in reducing the severe dengue burden in Bangladesh-A perspective.Front Microbiol. 2024 Aug 20;15:1423044. doi: 10.3389/fmicb.2024.1423044. eCollection 2024. Front Microbiol. 2024. PMID: 39228383 Free PMC article. Review.
-
Current Dengue Virus Vaccine Developments and Future Directions.Viruses. 2025 Jan 31;17(2):212. doi: 10.3390/v17020212. Viruses. 2025. PMID: 40006967 Free PMC article. Review.
References
-
- Hadinegoro S.R., Arredondo-García J.L., Capeding M.R., Deseda C., Chotpitayasunondh T., Dietze R., Muhammad Ismail H.I., Reynales H., Limkittikul K., Rivera-Medina D.M., et al. Efficacy and Long-Term Safety of a Dengue Vaccine in Regions of Endemic Disease. N. Engl. J. Med. 2015;373:1195–1206. doi: 10.1056/NEJMoa1506223. - DOI - PubMed
LinkOut - more resources
Full Text Sources

