A SARS-CoV-2 Vaccine Designed for Manufacturability Results in Unexpected Potency and Non-Waning Humoral Response
- PMID: 37112744
- PMCID: PMC10145385
- DOI: 10.3390/vaccines11040832
A SARS-CoV-2 Vaccine Designed for Manufacturability Results in Unexpected Potency and Non-Waning Humoral Response
Abstract
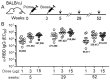
The rapid development of several highly efficacious SARS-CoV-2 vaccines was an unprecedented scientific achievement that saved millions of lives. However, now that SARS-CoV-2 is transitioning to the endemic stage, there exists an unmet need for new vaccines that provide durable immunity and protection against variants and can be more easily manufactured and distributed. Here, we describe a novel protein component vaccine candidate, MT-001, based on a fragment of the SARS-CoV-2 spike protein that encompasses the receptor binding domain (RBD). Mice and hamsters immunized with a prime-boost regimen of MT-001 demonstrated extremely high anti-spike IgG titers, and remarkably this humoral response did not appreciably wane for up to 12 months following vaccination. Further, virus neutralization titers, including titers against variants such as Delta and Omicron BA.1, remained high without the requirement for subsequent boosting. MT-001 was designed for manufacturability and ease of distribution, and we demonstrate that these attributes are not inconsistent with a highly immunogenic vaccine that confers durable and broad immunity to SARS-CoV-2 and its emerging variants. These properties suggest MT-001 could be a valuable new addition to the toolbox of SARS-CoV-2 vaccines and other interventions to prevent infection and curtail additional morbidity and mortality from the ongoing worldwide pandemic.
Keywords: COVID-19; SARS-CoV-2; durable immunity; emerging variants; protection; vaccine.
Conflict of interest statement
Authors EC and SA are co-founders and shareholders of Macrotope, Inc. and are named as inventors on patent applications describing MT-001 filed by Rutgers University. The remaining authors declare no competing interests.
Figures




References
-
- Hodcroft E.B. CoVariants: SARS-CoV-2 Mutations and Variants of Interest. 2021. [(accessed on 1 July 2021)]. Available online: https://covariants.org/
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

