Long-Term Follow-Up after Adoptive Transfer of BK-Virus-Specific T Cells in Hematopoietic Stem Cell Transplant Recipients
- PMID: 37112757
- PMCID: PMC10141379
- DOI: 10.3390/vaccines11040845
Long-Term Follow-Up after Adoptive Transfer of BK-Virus-Specific T Cells in Hematopoietic Stem Cell Transplant Recipients
Abstract
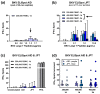
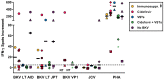
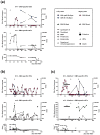
The BK virus (BKV) causes severe hemorrhagic cystitis in hematopoietic stem cell transplant (HSCT) recipients. To eliminate reactivated BKV, symptomatic patients can be treated with a reduction of the immunosuppressive therapy, with the antiviral drug cidofovir, or with virus-specific T cells (VSTs). In the current study, we compared the effect of VSTs to other treatment options, following up specific T cells using interferon-gamma ELISpot assay. We observed BKV large T-specific cellular responses in 12 out of 17 HSCT recipients with BKV-related cystitis (71%). In recipients treated with VSTs, 6 out of 7 showed specific T-cell responses, and that number in those without VSTs was 6 out of 10. In comparison, 27 out of 50 healthy controls (54%) responded. In HSCT recipients treated for BKV-related cystitis, absolute CD4+ T-cell numbers and renal function correlated with BKV-specific cellular responses (p = 0.03 and 0.01, respectively). In one patient, BKV-specific cellular immunity could already be detected at baseline, on day 35 after HSCT and prior to VSTs, and remained increased until day 226 after VSTs (78 vs. 7 spots increment). In conclusion, the ELISpot appears to be suitable to sensitively monitor BKV-specific cellular immunity in HSCT recipients, even early after transplantation or in the long term after VSTs.
Keywords: BK virus; CMV; EBV; ELISpot; JC virus; cidofovir; hematopoietic stem cell transplantation; immunosuppression; monitoring of T-cell immunity; treatment with virus-specific T cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Off-the-Shelf Virus-Specific T Cells to Treat BK Virus, Human Herpesvirus 6, Cytomegalovirus, Epstein-Barr Virus, and Adenovirus Infections After Allogeneic Hematopoietic Stem-Cell Transplantation.J Clin Oncol. 2017 Nov 1;35(31):3547-3557. doi: 10.1200/JCO.2017.73.0655. Epub 2017 Aug 7. J Clin Oncol. 2017. PMID: 28783452 Free PMC article. Clinical Trial.
-
Treatment of BK virus-associated hemorrhagic cystitis in pediatric hematopoietic stem cell transplant recipients with cidofovir: a single-center experience.Transpl Infect Dis. 2013 Dec;15(6):569-74. doi: 10.1111/tid.12136. Epub 2013 Sep 13. Transpl Infect Dis. 2013. PMID: 24028353
-
BK Virus Epidemiology, Risk Factors, and Clinical Outcomes: An Analysis of Hematopoietic Stem Cell Transplant Patients at Texas Children's Hospital.J Pediatric Infect Dis Soc. 2021 Apr 30;10(4):492-501. doi: 10.1093/jpids/piaa147. J Pediatric Infect Dis Soc. 2021. PMID: 33416086
-
BK virus infection in transplant recipients: clinical manifestations, treatment options and the immune response.Neth J Med. 2012 May;70(4):172-83. Neth J Med. 2012. PMID: 22641625 Review.
-
Beyond antivirals: virus-specific T-cell immunotherapy for BK virus haemorrhagic cystitis and JC virus progressive multifocal leukoencephalopathy.Curr Opin Infect Dis. 2021 Dec 1;34(6):627-634. doi: 10.1097/QCO.0000000000000794. Curr Opin Infect Dis. 2021. PMID: 34751182 Review.
Cited by
-
Polyomaviruses After Allogeneic Hematopoietic Stem Cell Transplantation.Viruses. 2025 Mar 12;17(3):403. doi: 10.3390/v17030403. Viruses. 2025. PMID: 40143330 Free PMC article. Review.
-
BK viral infection: A review of management and treatment.World J Transplant. 2023 Dec 18;13(6):309-320. doi: 10.5500/wjt.v13.i6.309. World J Transplant. 2023. PMID: 38174153 Free PMC article. Review.
-
BK Virus-Specific T Cell Response Associated with HLA Genotypes, RhD Status, and CMV or EBV Serostatus in Healthy Donors for Optimized Cell Therapy.J Clin Immunol. 2025 Jun 19;45(1):109. doi: 10.1007/s10875-025-01901-2. J Clin Immunol. 2025. PMID: 40537681 Free PMC article.
-
Donor Variability and PD-1 Expression Limit BK Polyomavirus-specific T-cell Function and Therapy.Transplantation. 2025 Sep 1;109(9):1526-1539. doi: 10.1097/TP.0000000000005399. Epub 2025 Apr 9. Transplantation. 2025. PMID: 40200394 Free PMC article.
References
-
- Saade A., Gras J., Darmon M., Michonneau D., Dhedin N., Feghoul L., Le Goff J., Xhaard A., De Latour R.P., Socie G., et al. Incidence, risk factors and outcome of BK virus hemorrhagic cystitis following allogenic hematopoietic cell transplantation: A retrospective cohort study. Bone Marrow Transpl. 2022;57:1287–1294. doi: 10.1038/s41409-022-01665-y. - DOI - PubMed
-
- Wong A.S., Cheng V.C., Yuen K.Y., Kwong Y.L., Leung A.Y. High frequency of polyoma BK virus shedding in the gastrointestinal tract after hematopoietic stem cell transplantation: A prospective and quantitative analysis. Bone Marrow Transpl. 2009;43:43–47. doi: 10.1038/bmt.2008.266. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

