SARS-CoV-2: Immunity, Challenges with Current Vaccines, and a Novel Perspective on Mucosal Vaccines
- PMID: 37112761
- PMCID: PMC10143972
- DOI: 10.3390/vaccines11040849
SARS-CoV-2: Immunity, Challenges with Current Vaccines, and a Novel Perspective on Mucosal Vaccines
Abstract
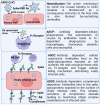
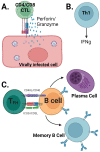
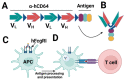
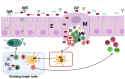
The global rollout of COVID-19 vaccines has played a critical role in reducing pandemic spread, disease severity, hospitalizations, and deaths. However, the first-generation vaccines failed to block severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and transmission, partially due to the limited induction of mucosal immunity, leading to the continuous emergence of variants of concern (VOC) and breakthrough infections. To meet the challenges from VOC, limited durability, and lack of mucosal immune response of first-generation vaccines, novel approaches are being investigated. Herein, we have discussed the current knowledge pertaining to natural and vaccine-induced immunity, and the role of the mucosal immune response in controlling SARS-CoV2 infection. We have also presented the current status of the novel approaches aimed at eliciting both mucosal and systemic immunity. Finally, we have presented a novel adjuvant-free approach to elicit effective mucosal immunity against SARS-CoV-2, which lacks the safety concerns associated with live-attenuated vaccine platforms.
Keywords: APC targeting; COVID-19; SARS-CoV-2; adjuvant; human FcγRI; immunity; intranasal vaccine; mucosal vaccine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Host Protective Immunity against Severe Acute Respiratory Coronavirus 2 (SARS-CoV-2) and the COVID-19 Vaccine-Induced Immunity against SARS-CoV-2 and Its Variants.Viruses. 2022 Nov 17;14(11):2541. doi: 10.3390/v14112541. Viruses. 2022. PMID: 36423150 Free PMC article. Review.
-
An intranasal vaccine targeting the receptor binding domain of SARS-CoV-2 elicits a protective immune response.Front Immunol. 2022 Nov 16;13:1005321. doi: 10.3389/fimmu.2022.1005321. eCollection 2022. Front Immunol. 2022. PMID: 36466882 Free PMC article.
-
Intranasal administration of a single dose of MVA-based vaccine candidates against COVID-19 induced local and systemic immune responses and protects mice from a lethal SARS-CoV-2 infection.Front Immunol. 2022 Sep 12;13:995235. doi: 10.3389/fimmu.2022.995235. eCollection 2022. Front Immunol. 2022. PMID: 36172368 Free PMC article.
-
An intranasal lentiviral booster reinforces the waning mRNA vaccine-induced SARS-CoV-2 immunity that it targets to lung mucosa.Mol Ther. 2022 Sep 7;30(9):2984-2997. doi: 10.1016/j.ymthe.2022.04.016. Epub 2022 Apr 27. Mol Ther. 2022. PMID: 35484842 Free PMC article.
-
Insights into COVID-19 Vaccine Development Based on Immunogenic Structural Proteins of SARS-CoV-2, Host Immune Responses, and Herd Immunity.Cells. 2021 Oct 29;10(11):2949. doi: 10.3390/cells10112949. Cells. 2021. PMID: 34831172 Free PMC article. Review.
Cited by
-
SARS-CoV-2 Vaccines: The Advantage of Mucosal Vaccine Delivery and Local Immunity.Vaccines (Basel). 2024 Jul 18;12(7):795. doi: 10.3390/vaccines12070795. Vaccines (Basel). 2024. PMID: 39066432 Free PMC article. Review.
-
Updated Considerations for the Immunopharmacological Aspects of the "Talented mRNA Vaccines".Vaccines (Basel). 2023 Sep 12;11(9):1481. doi: 10.3390/vaccines11091481. Vaccines (Basel). 2023. PMID: 37766157 Free PMC article. Review.
-
Safety and Immunogenicity of the Live Attenuated Vaccine QazCOVID-Live Against Coronavirus Infection COVID-19: Pre-Clinical Study Results.Vaccines (Basel). 2024 Dec 12;12(12):1401. doi: 10.3390/vaccines12121401. Vaccines (Basel). 2024. PMID: 39772061 Free PMC article.
-
Sex-biased immunogenicity of a mucosal subunit vaccine against SARS-CoV-2 in mice.Front Immunol. 2024 May 21;15:1386243. doi: 10.3389/fimmu.2024.1386243. eCollection 2024. Front Immunol. 2024. PMID: 38835757 Free PMC article.
-
A multi-epitope/CXCL11 prime/pull coronavirus mucosal vaccine boosts the frequency and the function of lung-resident memory CD4+ and CD8+ T cells and enhanced protection against COVID-19-like symptoms and death caused by SARS-CoV-2 infection.J Virol. 2023 Dec 21;97(12):e0109623. doi: 10.1128/jvi.01096-23. Epub 2023 Dec 1. J Virol. 2023. PMID: 38038432 Free PMC article.
References
-
- Brouwer P.J.M., Caniels T.G., van der Straten K., Snitselaar J.L., Aldon Y., Bangaru S., Torres J.L., Okba N.M.A., Claireaux M., Kerster G., et al. Potent neutralizing antibodies from COVID-19 patients define multiple targets of vulnerability. Science. 2020;369:643–650. doi: 10.1126/science.abc5902. - DOI - PMC - PubMed
-
- The COVID-19 Vaccine Tracker and Landscape. [(accessed on 3 March 2023)]. Available online: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-cand....
-
- COVID-19 Vaccination Data. [(accessed on 3 March 2023)]. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19....
-
- Ella R., Reddy S., Blackwelder W., Potdar V., Yadav P., Sarangi V., Aileni V.K., Kanungo S., Rai S., Reddy P., et al. Efficacy, safety, and lot-to-lot immunogenicity of an inactivated SARS-CoV-2 vaccine (BBV152): Interim results of a randomised, double-blind, controlled, phase 3 trial. Lancet. 2021;398:2173–2184. doi: 10.1016/S0140-6736(21)02000-6. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

