Effectiveness, Immunogenicity and Harms of Additional SARS-CoV-2 Vaccine Doses in Kidney Transplant Recipients: A Systematic Review
- PMID: 37112775
- PMCID: PMC10141039
- DOI: 10.3390/vaccines11040863
Effectiveness, Immunogenicity and Harms of Additional SARS-CoV-2 Vaccine Doses in Kidney Transplant Recipients: A Systematic Review
Abstract
Background: Kidney transplant recipients (KTRs) who have a highly impaired immune response are in need of intensified and safe vaccination strategies to achieve seroconversion and prevent severe disease.
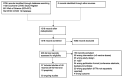
Methods: We searched the Web of Science Core Collection, the Cochrane COVID-19 Study Register and the WHO COVID-19 global literature on coronavirus disease from January 2020 to 22 July 2022 for prospective studies that assessed immunogenicity and efficacy after three or more SARS-CoV-2 vaccine doses.
Results: In 37 studies on 3429 patients, de novo seroconversion after three and four vaccine doses ranged from 32 to 60% and 25 to 37%. Variant-specific neutralization was 59 to 70% for Delta and 12 to 52% for Omicron. Severe disease after infection was rarely reported but all concerned KTRs lacked immune responses after vaccination. Studies investigating the clinical course of COVID-19 found remarkably higher rates of severe disease than in the general population. Serious adverse events and acute graft rejections were very rare. Substantial heterogeneity between the studies limited their comparability and summary.
Conclusion: Additional SARS-CoV-2 vaccine doses are potent and safe in general terms as well as regarding transplant-specific outcomes whilst the Omicron wave remains a significant threat to KTRs without adequate immune responses.
Keywords: COVID-19; SARS-CoV-2 vaccines; acute graft rejection; kidney transplant recipients.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Alternative strategies to increase the immunogenicity of COVID-19 vaccines in kidney transplant recipients not responding to two or three doses of an mRNA vaccine (RECOVAC): a randomised clinical trial.Lancet Infect Dis. 2023 Mar;23(3):307-319. doi: 10.1016/S1473-3099(22)00650-8. Epub 2022 Oct 27. Lancet Infect Dis. 2023. PMID: 36354032 Free PMC article. Clinical Trial.
-
Comparison of SARS-CoV-2 Antibody Response 4 Weeks After Homologous vs Heterologous Third Vaccine Dose in Kidney Transplant Recipients: A Randomized Clinical Trial.JAMA Intern Med. 2022 Feb 1;182(2):165-171. doi: 10.1001/jamainternmed.2021.7372. JAMA Intern Med. 2022. PMID: 34928302 Free PMC article. Clinical Trial.
-
Neutralizing antibody response against the B.1.617.2 (delta) and the B.1.1.529 (omicron) variants after a third mRNA SARS-CoV-2 vaccine dose in kidney transplant recipients.Am J Transplant. 2022 Jul;22(7):1873-1883. doi: 10.1111/ajt.17054. Epub 2022 Apr 18. Am J Transplant. 2022. PMID: 35384272 Free PMC article.
-
Seroconversion rates in kidney transplant recipients following SARS-CoV-2 vaccination and its association with immunosuppressive agents: a systematic review and meta-analysis.Clin Exp Vaccine Res. 2023 Jan;12(1):13-24. doi: 10.7774/cevr.2023.12.1.13. Epub 2023 Jan 31. Clin Exp Vaccine Res. 2023. PMID: 36844682 Free PMC article. Review.
-
SARS-CoV-2 Vaccines: Safety and Immunogenicity in Solid Organ Transplant Recipients and Strategies for Improving Vaccine Responses.Curr Transplant Rep. 2022;9(1):35-47. doi: 10.1007/s40472-022-00359-0. Epub 2022 Jan 22. Curr Transplant Rep. 2022. PMID: 35096509 Free PMC article. Review.
Cited by
-
Humoral immunity to SARS-CoV-2 in kidney transplant recipients and dialysis patients: IgA and IgG patterns unraveled after SARS-CoV-2 infection and vaccination.Virol J. 2024 Jun 13;21(1):138. doi: 10.1186/s12985-024-02410-1. Virol J. 2024. PMID: 38872127 Free PMC article.
References
-
- Nyberg T., Ferguson N.M., Nash S.G., Webster H.H., Flaxman S., Andrews N., Hinsley W., Bernal J.L., Kall M., Bhatt S., et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: A cohort study. Lancet. 2022;399:1303–1312. doi: 10.1016/S0140-6736(22)00462-7. - DOI - PMC - PubMed
-
- Külper-Schiek W., Piechotta V., Pilic A., Batke M., Dreveton L.-S., Geurts B., Koch J., Köppe S., Treskova M., Vygen-Bonnet S., et al. Facing the Omicron variant—How well do vaccines protect against mild and severe COVID-19? Third interim analysis of a living systematic review. Front. Immunol. 2022;13:940562. doi: 10.3389/fimmu.2022.940562. - DOI - PMC - PubMed
-
- Herman G., O’Brien M.P., Forleo-Neto E., Sarkar N., Isa F., Hou P., Chan K.-C., Bar K.J., Barnabas R.V., Barouch D.H., et al. Efficacy and safety of a single dose of casirivimab and imdevimab for the prevention of COVID-19 over an 8-month period: A randomised, double-blind, placebo-controlled trial. Lancet Infect. Dis. 2022;22:1444–1454. doi: 10.1016/S1473-3099(22)00416-9. - DOI - PMC - PubMed
-
- Administration USFaD Updated 29 June 2022. [(accessed on 1 October 2022)];2022 Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-authorizes-re....
-
- Benotmane I., Velay A., Gautier-Vargas G., Olagne J., Obrecht A., Cognard N., Heibel F., Braun-Parvez L., Keller N., Martzloff J., et al. Breakthrough COVID-19 cases despite prophylaxis with 150 mg of tixagevimab and 150 mg of cilgavimab in kidney transplant recipients. Am. J. Transplant. 2022;22:2675–2681. doi: 10.1111/ajt.17121. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

