SARS-CoV-2 Neutralizing Antibodies to B.1 and to BA.5 Variant after Booster Dose of BNT162b2 Vaccine in HIV Patients COVID-Naïve and on Successful Antiretroviral Therapy
- PMID: 37112782
- PMCID: PMC10144758
- DOI: 10.3390/vaccines11040871
SARS-CoV-2 Neutralizing Antibodies to B.1 and to BA.5 Variant after Booster Dose of BNT162b2 Vaccine in HIV Patients COVID-Naïve and on Successful Antiretroviral Therapy
Abstract
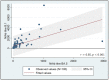
Live virus neutralization is the gold standard to investigate immunity. This prospective observational study aimed to determine the magnitude of response against the original B.1 lineage and against the BA.5 lineage six months after the third BNT162b2 mRNA vaccine dose in patients with HIV infection on successful antiretroviral treatment and no previous SARS-CoV-2 infection. A total of 100 subjects (M/F 83/17, median age 54 years) were included in the analysis: 95 had plasma HIV RNA <40 copies/mL, the median CD4+ T cell count at the administration of the third dose was 580 cells/mm3, and the median nadir CD4+ T cell count was 258 cells/mm3. Neutralizing antibodies (NtAb) against B.1 were detectable in all the subjects, but those to BA.5 were only detected in 88 (p < 0.001). The median NtAb titer to B.1 was significantly higher than that to BA.5 (393 vs. 60, p < 0.0001), and there was a strong positive correlation between the paired measurements (p < 0.0001). Linear regression on a subset of 87 patients excluding outlier NtAb titers showed that 48% of the changes in NtAb titers to BA.5 are related to the changes in value titers to B.1. SARS-CoV-2 variants evolve rapidly, challenging the efficacy of vaccines, and data on comparative NtAb responses may help in tailoring intervals between vaccine doses and in predicting vaccine efficacy.
Keywords: B.1 lineage; BA.5 lineage; BNT162b2 mRNA vaccine; HIV; SARS-CoV-2; neutralizing antibodies; third dose.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analysis, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

References
-
- [(accessed on 7 November 2022)]. Available online: https://www.iss.it/web/guest/-/bollettino-varianti-del-28-ottobre-2022.
-
- Tartof S.Y., Slezak J.M., Puzniak L., Hong V., Frankland T.B., Ackerson B.K., Takhar H., Ogun O.A., Simmons S., Zamparo J.M., et al. BNT162b2 vaccine effectiveness against SARS-CoV-2 omicron BA.4 and BA.5. Lancet Infect. Dis. 2022;22:1663–1665. doi: 10.1016/S1473-3099(22)00692-2. - DOI - PMC - PubMed
-
- Cheng S.S., Mok C.K., Li J.K., Ng S.S., Lam B.H., Jeevan T., Kandeil A., Pekosz A., Chan K.C., Tsang L.C., et al. Plaque-neutralizing antibody to BA.2.12.1, BA.4 and BA.5 in individuals with three doses of BioNTech or CoronaVac vaccines, natural infection and breakthrough infection. J. Clin. Virol. 2022;156:105273. doi: 10.1016/j.jcv.2022.105273. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

