The Complex Role of HBeAg and Its Precursors in the Pathway to Hepatocellular Carcinoma
- PMID: 37112837
- PMCID: PMC10144019
- DOI: 10.3390/v15040857
The Complex Role of HBeAg and Its Precursors in the Pathway to Hepatocellular Carcinoma
Abstract
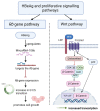
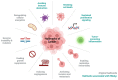
Hepatitis B virus (HBV) is one of the seven known human oncogenic viruses and has adapted to coexist with a single host for prolonged periods, requiring continuous manipulation of immunity and cell fate decisions. The persistence of HBV infection is associated with the pathogenesis of hepatocellular carcinoma, and various HBV proteins have been implicated in promoting this persistence. The precursor of hepatitis e antigen (HBeAg), is translated from the precore/core region and is post-translationally modified to yield HBeAg, which is secreted in the serum. HBeAg is a non-particulate protein of HBV and can act as both a tolerogen and an immunogen. HBeAg can protect hepatocytes from apoptosis by interfering with host signalling pathways and acting as a decoy to the immune response. By evading the immune response and interfering with apoptosis, HBeAg has the potential to contribute to the hepatocarcinogenic potential of HBV. In particular, this review summarises the various signalling pathways through which HBeAg and its precursors can promote hepatocarcinogenesis via the various hallmarks of cancer.
Keywords: HBeAg; hepatitis B virus; hepatocellular carcinoma.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- WHO . Hepatitis B Vaccine Epidemology Record. WHO; Geneva, Switzerland: 2019.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

