Viral Protein VP1 Virus-like Particles (VLP) of CVB4 Induces Protective Immunity against Lethal Challenges with Diabetogenic E2 and Wild Type JBV Strains in Mice Model
- PMID: 37112858
- PMCID: PMC10145976
- DOI: 10.3390/v15040878
Viral Protein VP1 Virus-like Particles (VLP) of CVB4 Induces Protective Immunity against Lethal Challenges with Diabetogenic E2 and Wild Type JBV Strains in Mice Model
Abstract
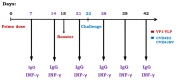
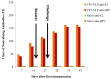
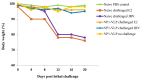
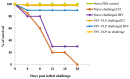
Several epidemiological studies demonstrated that coxsackievirus B4 (CVB4) causes viral pancreatitis and can ultimately result in type 1 diabetes mellitus (T1D). Prevention of CVB4 infection is therefore highly desirable. There is currently no vaccine or antiviral therapeutic reagent in clinical use. VLP are structurally similar to native virus particles and therefore are far better immunogens than any other subunit vaccines. Many studies have shown the potential of capsid protein VP1 on providing protective effects from different viral strains. In this study, we contributed towards the development of a CVB4 VLP-based vaccine from the total protein VP1 of the diabetogenic CVB4E2 strain and assessed whether it could induce a protective immunity against both the wild-type CVB4JBV and the diabetogenic CVB4E2 strains in mice model. Serum samples, taken from mice immunized with VLP, were assayed in vitro for their anti-CVB4 neutralizing activity and in vivo for protective activity. We show that VLP vaccine generates robust immune responses that protect mice from lethal challenges. Results demonstrate that CVB4 VP1 capsid proteins expressed in insect cells have the intrinsic capacity to assemble into non-infectious VLP, which afforded protection from CVB4 infection to mice when used as a vaccine.
Keywords: coxsackievirus B4; immunization; lethal challenge; type 1 diabetes; vaccine; viral protein VP1; virus-like particles.
Conflict of interest statement
The manuscript is an original work and has not been published or is under consideration for publication in another journal. The study complies with current ethical considerations. The authors confirm that all the listed authors have participated actively in the study and have seen and approved the submitted manuscript. The authors do not have any possible conflict of interest.
Figures



Similar articles
-
Immunogenicity and protective efficacy of inactivated coxsackievirus B4 viral particles.Emerg Microbes Infect. 2024 Dec;13(1):2337665. doi: 10.1080/22221751.2024.2337665. Epub 2024 Apr 5. Emerg Microbes Infect. 2024. PMID: 38551145 Free PMC article.
-
Vaccination with coxsackievirus B3 virus-like particles elicits humoral immune response and protects mice against myocarditis.Vaccine. 2012 Mar 16;30(13):2301-8. doi: 10.1016/j.vaccine.2012.01.061. Epub 2012 Jan 31. Vaccine. 2012. PMID: 22306858
-
Characterization of Coxsackievirus B4 virus-like particles VLP produced by the recombinant baculovirus-insect cell system expressing the major capsid protein.Mol Biol Rep. 2020 Apr;47(4):2835-2843. doi: 10.1007/s11033-020-05333-6. Epub 2020 Apr 2. Mol Biol Rep. 2020. PMID: 32240468
-
A virus-like particle vaccine protects mice against coxsackievirus A10 lethal infection.Antiviral Res. 2018 Apr;152:124-130. doi: 10.1016/j.antiviral.2018.02.016. Epub 2018 Feb 20. Antiviral Res. 2018. PMID: 29470994
-
Developments in L2-based human papillomavirus (HPV) vaccines.Virus Res. 2017 Mar 2;231:166-175. doi: 10.1016/j.virusres.2016.11.020. Epub 2016 Nov 23. Virus Res. 2017. PMID: 27889616 Free PMC article. Review.
Cited by
-
T-Cell Receptor Sequences Identify Combined Coxsackievirus-Streptococci Infections as Triggers for Autoimmune Myocarditis and Coxsackievirus-Clostridia Infections for Type 1 Diabetes.Int J Mol Sci. 2024 Feb 1;25(3):1797. doi: 10.3390/ijms25031797. Int J Mol Sci. 2024. PMID: 38339075 Free PMC article.
-
Immunogenicity and protective efficacy of inactivated coxsackievirus B4 viral particles.Emerg Microbes Infect. 2024 Dec;13(1):2337665. doi: 10.1080/22221751.2024.2337665. Epub 2024 Apr 5. Emerg Microbes Infect. 2024. PMID: 38551145 Free PMC article.
-
Research progress and application prospects of animal models of group B Coxsackievirus infections.Emerg Microbes Infect. 2025 Dec;14(1):2441391. doi: 10.1080/22221751.2024.2441391. Epub 2024 Dec 22. Emerg Microbes Infect. 2025. PMID: 39665300 Free PMC article. Review.
References
-
- Norris J.M., Dorman J.S., Rezers M., Porte R.E. The epidemiology and genetics of insulindependent diabetes mellitus. Arch. Pathol. Lab. Med. 1987;111:905–909. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

