In Vitro Investigation of the Interaction of Avian Metapneumovirus and Newcastle Disease Virus with Turkey Respiratory and Reproductive Tissue
- PMID: 37112886
- PMCID: PMC10144051
- DOI: 10.3390/v15040907
In Vitro Investigation of the Interaction of Avian Metapneumovirus and Newcastle Disease Virus with Turkey Respiratory and Reproductive Tissue
Abstract
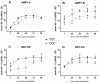
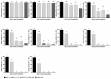
In poultry, several respiratory viral infections lead to a drop in egg production associated with high economic losses. While the virus-host interactions at the respiratory epithelium are well studied, less is known about these interactions in the oviduct. To investigate possible differences between virus infections at these epithelial structures, we compared the interactions of two important poultry viruses on turkey organ cultures. Two members of the order Mononegavirales, the Avian Metapneumovirus (AMPV) and the Newcastle disease virus (NDV), were selected to conduct the in vitro experiments since these viruses can infect both the trachea and oviduct. In addition, we used different strains of these viruses, a subtype A and a subtype B strain for AMPV and the NDV strains Komarow and Herts'33, to detect possible differences not only between the tissues but also between different viral strains. Turkey tracheal and oviduct organ cultures (TOC and OOC) were prepared to investigate viral replication, antigen localisation, lesion development, and the expression pattern of interferon-λ and importin-α isoforms. All viruses replicated more efficiently in the oviduct than in the tracheal epithelium (p < 0.05). In addition, we observed higher expression levels of both, IFN-λ and importin-α in OOCs compared to TOCs. Our results indicated strain-dependent differences, with the AMPV-B- and Herts'33 strains being more virulent in organ cultures than the AMPV-A- and Komarow strains, based on the higher viral genome loads, more severe histological lesions, and higher upregulation of IFN-λ. Overall, our findings reveal tissue- and virus strain-dependent differences, which may have consequences for disease development in the host tissue and, subsequently, possible treatment strategies.
Keywords: avian metapneumovirus; importin-alpha; newcastle disease virus; oviduct organ culture (OOC); tracheal organ culture (TOC); virus–host interactions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Avian metapneumovirus infection of chicken and turkey tracheal organ cultures: comparison of virus-host interactions.Avian Pathol. 2015;44(6):480-9. doi: 10.1080/03079457.2015.1086974. Avian Pathol. 2015. PMID: 26365279
-
Methyltransferase-defective avian metapneumovirus vaccines provide complete protection against challenge with the homologous Colorado strain and the heterologous Minnesota strain.J Virol. 2014 Nov;88(21):12348-63. doi: 10.1128/JVI.01095-14. Epub 2014 Aug 13. J Virol. 2014. PMID: 25122790 Free PMC article.
-
Generation and evaluation of a recombinant Newcastle disease virus expressing the glycoprotein (G) of avian metapneumovirus subgroup C as a bivalent vaccine in turkeys.Vaccine. 2011 Nov 3;29(47):8624-33. doi: 10.1016/j.vaccine.2011.09.007. Epub 2011 Sep 14. Vaccine. 2011. PMID: 21925228
-
Immune responses of poultry to Newcastle disease virus.Dev Comp Immunol. 2013 Nov;41(3):447-53. doi: 10.1016/j.dci.2013.04.012. Epub 2013 Apr 25. Dev Comp Immunol. 2013. PMID: 23623955 Review.
-
Zoonotic Origins of Human Metapneumovirus: A Journey from Birds to Humans.Viruses. 2022 Mar 25;14(4):677. doi: 10.3390/v14040677. Viruses. 2022. PMID: 35458407 Free PMC article. Review.
Cited by
-
Effects of G and SH Truncation on the Replication, Virulence, and Immunogenicity of Avian Metapneumovirus.Vaccines (Basel). 2024 Jan 21;12(1):106. doi: 10.3390/vaccines12010106. Vaccines (Basel). 2024. PMID: 38276678 Free PMC article.
-
Pathological and phylogenetic characteristics of fowl AOAV-1 and H5 isolated from naturally infected Meleagris Gallopavo.BMC Vet Res. 2024 May 21;20(1):216. doi: 10.1186/s12917-024-04029-4. BMC Vet Res. 2024. PMID: 38773480 Free PMC article.
References
-
- Mork A.K., Hesse M., Abd El Rahman S., Rautenschlein S., Herrler G., Winter C. Differences in the tissue tropism to chicken oviduct epithelial cells between avian coronavirus IBV strains QX and B1648 are not related to the sialic acid binding properties of their spike proteins. Vet. Res. 2014;45:67. doi: 10.1186/1297-9716-45-67. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

