Multifactorial White Matter Damage in the Acute Phase and Pre-Existing Conditions May Drive Cognitive Dysfunction after SARS-CoV-2 Infection: Neuropathology-Based Evidence
- PMID: 37112888
- PMCID: PMC10144140
- DOI: 10.3390/v15040908
Multifactorial White Matter Damage in the Acute Phase and Pre-Existing Conditions May Drive Cognitive Dysfunction after SARS-CoV-2 Infection: Neuropathology-Based Evidence
Abstract
Background: There is an urgent need to better understand the mechanisms underlying acute and long-term neurological symptoms after COVID-19. Neuropathological studies can contribute to a better understanding of some of these mechanisms.
Methods: We conducted a detailed postmortem neuropathological analysis of 32 patients who died due to COVID-19 during 2020 and 2021 in Austria.
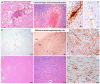
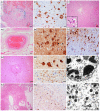
Results: All cases showed diffuse white matter damage with a diffuse microglial activation of a variable severity, including one case of hemorrhagic leukoencephalopathy. Some cases revealed mild inflammatory changes, including olfactory neuritis (25%), nodular brainstem encephalitis (31%), and cranial nerve neuritis (6%), which were similar to those observed in non-COVID-19 severely ill patients. One previously immunosuppressed patient developed acute herpes simplex encephalitis. Acute vascular pathologies (acute infarcts 22%, vascular thrombosis 12%, diffuse hypoxic-ischemic brain damage 40%) and pre-existing small vessel diseases (34%) were frequent findings. Moreover, silent neurodegenerative pathologies in elderly persons were common (AD neuropathologic changes 32%, age-related neuronal and glial tau pathologies 22%, Lewy bodies 9%, argyrophilic grain disease 12.5%, TDP43 pathology 6%).
Conclusions: Our results support some previous neuropathological findings of apparently multifactorial and most likely indirect brain damage in the context of SARS-CoV-2 infection rather than virus-specific damage, and they are in line with the recent experimental data on SARS-CoV-2-related diffuse white matter damage, microglial activation, and cytokine release.
Keywords: COVID-19; SARS-CoV-2; leukoencephalopathy; neuropathology; white matter.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

Similar articles
-
COVID-19-related neuropathology and microglial activation in elderly with and without dementia.Brain Pathol. 2021 Sep;31(5):e12997. doi: 10.1111/bpa.12997. Epub 2021 Jun 18. Brain Pathol. 2021. PMID: 34145669 Free PMC article.
-
Neuroimaging Patterns in Patients with COVID-19-Associated Neurological Complications: A Review.Neurol India. 2021 Mar-Apr;69(2):260-271. doi: 10.4103/0028-3886.314531. Neurol India. 2021. PMID: 33904434 Review.
-
Brain autopsies of critically ill COVID-19 patients demonstrate heterogeneous profile of acute vascular injury, inflammation and age-linked chronic brain diseases.Acta Neuropathol Commun. 2022 Dec 17;10(1):186. doi: 10.1186/s40478-022-01493-7. Acta Neuropathol Commun. 2022. PMID: 36528671 Free PMC article.
-
Neuropathology of COVID-19: a spectrum of vascular and acute disseminated encephalomyelitis (ADEM)-like pathology.Acta Neuropathol. 2020 Jul;140(1):1-6. doi: 10.1007/s00401-020-02166-2. Epub 2020 May 24. Acta Neuropathol. 2020. PMID: 32449057 Free PMC article.
-
Neurological manifestations associated with SARS-CoV-2 and other coronaviruses: A narrative review for clinicians.Rev Neurol (Paris). 2021 Jan-Feb;177(1-2):51-64. doi: 10.1016/j.neurol.2020.10.001. Epub 2020 Dec 16. Rev Neurol (Paris). 2021. PMID: 33446327 Free PMC article. Review.
Cited by
-
Proteomic and transcriptomic profiling of brainstem, cerebellum and olfactory tissues in early- and late-phase COVID-19.Nat Neurosci. 2024 Mar;27(3):409-420. doi: 10.1038/s41593-024-01573-y. Epub 2024 Feb 16. Nat Neurosci. 2024. PMID: 38366144
-
Neuropathology in COVID-19 autopsies is defined by microglial activation and lesions of the white matter with emphasis in cerebellar and brain stem areas.Front Neurol. 2023 Jul 13;14:1229641. doi: 10.3389/fneur.2023.1229641. eCollection 2023. Front Neurol. 2023. PMID: 37521293 Free PMC article.
-
The neurobiology of SARS-CoV-2 infection.Nat Rev Neurosci. 2024 Jan;25(1):30-42. doi: 10.1038/s41583-023-00769-8. Epub 2023 Dec 4. Nat Rev Neurosci. 2024. PMID: 38049610 Review.
-
"Spanish flu," encephalitis lethargica, and COVID-19: Progress made, lessons learned, and directions for future research.Eur J Neurol. 2024 Nov;31(11):e16312. doi: 10.1111/ene.16312. Epub 2024 May 14. Eur J Neurol. 2024. PMID: 38745394 Free PMC article. Review.
-
On the merits and potential of advanced neuroimaging techniques in COVID-19: A scoping review.Neuroimage Clin. 2024;42:103589. doi: 10.1016/j.nicl.2024.103589. Epub 2024 Mar 6. Neuroimage Clin. 2024. PMID: 38461701 Free PMC article.
References
-
- Travi G., Rossotti R., Merli M., D’Amico F., Chiappetta S., Giussani G., Panariello A., Corradin M., Vecchi M., Raimondi A., et al. Neurological manifestations in patients hospitalized with COVID-19: A retrospective analysis from a large cohort in Northern Italy. Eur. J. Neurosci. 2021;53:2912–2922. doi: 10.1111/ejn.15159. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

