Longitudinal Detection of Twenty DNA and RNA Viruses in Allogeneic Hematopoietic Stem Cell Transplant Recipients Plasma
- PMID: 37112908
- PMCID: PMC10142697
- DOI: 10.3390/v15040928
Longitudinal Detection of Twenty DNA and RNA Viruses in Allogeneic Hematopoietic Stem Cell Transplant Recipients Plasma
Abstract
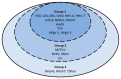
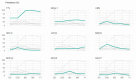
Metagenomics revealed novel and routinely overlooked viruses, representing sources of unrecognized infections after allogeneic hematopoietic stem cell transplantation (allo-HSCT). We aim to describe DNA and RNA virus prevalence and kinetics in allo-HSCT recipients' plasma for one year post HSCT. We included 109 adult patients with first allo-HSCT from 1 March 2017 to 31 January 2019 in this observational cohort study. Seventeen DNA and three RNA viral species were screened with qualitative and/or quantitative r(RT)-PCR assays using plasma samples collected at 0, 1, 3, 6, and 12 months post HSCT. TTV infected 97% of patients, followed by HPgV-1 (prevalence: 26-36%). TTV (median 3.29 × 105 copies/mL) and HPgV-1 (median 1.18 × 106 copies/mL) viral loads peaked at month 3. At least one Polyomaviridae virus (BKPyV, JCPyV, MCPyV, HPyV6/7) was detected in >10% of patients. HPyV6 and HPyV7 prevalence reached 27% and 12% at month 3; CMV prevalence reached 27%. HSV, VZV, EBV, HHV-7, HAdV and B19V prevalence remained <5%. HPyV9, TSPyV, HBoV, EV and HPg-V2 were never detected. At month 3, 72% of patients had co-infections. TTV and HPgV-1 infections were highly prevalent. BKPyV, MCPyV and HPyV6/7 were frequently detected relative to classical culprits. Further investigation is needed into associations between these viral infections and immune reconstitution or clinical outcomes.
Keywords: PCR; TTV; anellovirus; blood; human pegivirus; polyomavirus; transplantation; virome; virus.
Conflict of interest statement
The authors declare that they have no competing interest. Y.C.: consulting fees from MSD, Novartis, Incyte, BMS, Pfizer, Abbvie, Roche, Jazz, Gilead, Amgen, Astra-Zeneca, Servier; Travel support from MSD, Roche, Gilead, Amgen, Incyte, Abbvie, Janssen, Astra-Zeneca, Jazz.
Figures




References
-
- Vu D.L., Dayer J.A., Masouridi-Levrat S., Combescure C., Boely E., Khanna N., Mueller N.J., Kleber M., Medinger M., Halter J., et al. Microbiologically documented infections after adult allogeneic hematopoietic cell transplantation: A 5-year analysis within the Swiss Transplant Cohort study. Transpl. Infect. Dis. 2020;22:e13289. doi: 10.1111/tid.13289. - DOI - PubMed
-
- Vu D.L., Cordey S., Simonetta F., Brito F., Docquier M., Turin L., van Delden C., Boely E., Dantin C., Pradier A., et al. Human pegivirus persistence in human blood virome after allogeneic haematopoietic stem-cell transplantation. Clin. Microbiol. Infect. 2019;25:225–232. doi: 10.1016/j.cmi.2018.05.004. - DOI - PubMed
-
- Zanella M.C., Cordey S., Laubscher F., Docquier M., Vieille G., Van Delden C., Braunersreuther V., Ta M.K., Lobrinus J.A., Masouridi-Levrat S., et al. Unmasking viral sequences by metagenomic next-generation sequencing in adult human blood samples during steroid-refractory/dependent graft-versus-host disease. Microbiome. 2021;9:28. doi: 10.1186/s40168-020-00953-3. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

