Changes in the Etiology of Acute Respiratory Infections among Children in Novosibirsk, Russia, between 2019 and 2022: The Impact of the SARS-CoV-2 Virus
- PMID: 37112913
- PMCID: PMC10141072
- DOI: 10.3390/v15040934
Changes in the Etiology of Acute Respiratory Infections among Children in Novosibirsk, Russia, between 2019 and 2022: The Impact of the SARS-CoV-2 Virus
Abstract
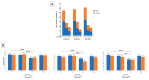
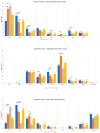
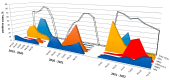
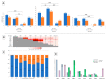
A wide range of human respiratory viruses are known that may cause acute respiratory infections (ARIs), such as influenza A and B viruses (HIFV), respiratory syncytial virus (HRSV), coronavirus (HCoV), parainfluenza virus (HPIV), metapneumovirus (HMPV), rhinovirus (HRV), adenovirus (HAdV), bocavirus (HBoV), and others. The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) caused the COronaVIrus Disease (COVID) that lead to pandemic in 2019 and significantly impacted on the circulation of ARIs. The aim of this study was to analyze the changes in the epidemic patterns of common respiratory viruses among children and adolescents hospitalized with ARIs in hospitals in Novosibirsk, Russia, from November 2019 to April 2022. During 2019 and 2022, nasal and throat swabs were taken from a total of 3190 hospitalized patients 0-17 years old for testing for HIFV, HRSV, HCoV, HPIV, HMPV, HRV, HAdV, HBoV, and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by real-time PCR. The SARS-CoV-2 virus dramatically influenced the etiology of acute respiratory infections among children and adolescents between 2019 and 2022. We observed dramatic changes in the prevalence of major respiratory viruses over three epidemic research seasons: HIFV, HRSV, and HPIV mainly circulated in 2019-2020; HMPV, HRV, and HCoV dominated in 2020-2021; and HRSV, SARS-CoV-2, HIFV, and HRV were the most numerous agents in 2021-2022. Interesting to note was the absence of HIFV and a significant reduction in HRSV during the 2020-2021 period, while HMPV was absent and there was a significant reduction of HCoV during the following epidemic period in 2021-2022. Viral co-infection was significantly more frequently detected in the 2020-2021 period compared with the other two epidemic seasons. Certain respiratory viruses, HCoV, HPIV, HBoV, HRV, and HAdV, were registered most often in co-infections. This cohort study has revealed that during the pre-pandemic and pandemic periods, there were dramatic fluctuations in common respiratory viruses registered among hospitalized patients 0-17 years old. The most dominant virus in each research period differed: HIFV in 2019-2020, HMPV in 2020-2021, and HRSV in 2021-2022. Virus-virus interaction was found to be possible between SARS-CoV-2 and HRV, HRSV, HAdV, HMPV, and HPIV. An increase in the incidence of COVID-19 was noted only during the third epidemic season (January to March 2022).
Keywords: HAdV; HBoV; HCoV; HIFV; HMPV; HPIV; HRSV; HRV; SARS-CoV-2.
Conflict of interest statement
The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Figures
References
-
- Lozano R., Naghavi M., Foreman K., Lim S., Shibuya K., Aboyans V., Abraham J., Adair T., Aggarwal R., Ahn S.Y., et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. - DOI - PMC - PubMed
-
- Kurskaya O., Ryabichenko T., Leonova N., Shi W., Bi H., Sharshov K., Kazachkova E., Sobolev I., Prokopyeva E., Kartseva T., et al. Viral etiology of Acute Respiratory Infections in Hospitalized Children in Novosibirsk City, Russia (2013–2017) PLoS ONE. 2018;13:e0200117. doi: 10.1371/journal.pone.0200117. - DOI - PMC - PubMed
-
- Varela F.H., Scotta M.C., Polese-Bonatto M., Sartor I.T.S., Ferreira C.F., Fernandes I.R., Zavaglia G.O., de Almeida W.A.F., Arakaki-Sanchez D., Pinto L.A., et al. Absence of detection of RSV and influenza during the COVID-19 pandemic in a Brazilian cohort: Likely role of lower transmission in the community. J. Glob. Health. 2021;11:05007. doi: 10.7189/jogh.11.05007. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

