Differences in Pathogenicity and Vaccine Resistance Discovered between Two Epidemic Strains of Marek's Disease Virus in China
- PMID: 37112925
- PMCID: PMC10145439
- DOI: 10.3390/v15040945
Differences in Pathogenicity and Vaccine Resistance Discovered between Two Epidemic Strains of Marek's Disease Virus in China
Abstract
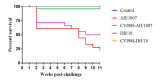
Despite highly effective vaccines, Marek's disease (MD) causes great economic loss to the poultry industry annually, largely due to the continuous emergence of new MD virus (MDV) strains. To explore the pathogenic characteristics of newly emerged MDV strains, we selected two strains (AH/1807 and DH/18) with clinically different pathotypes. We studied each strain's infection process and pathogenicity and observed differences in immunosuppression and vaccine resistance. Specific pathogen-free chickens, unvaccinated or vaccinated with CVI988, were challenged with AH/1807 or DH/18. Both infections induced MD damage; however, differences were observed in terms of mortality (AH/1807: 77.8%, DH/18: 50%) and tumor rates (AH/1807: 50%, DH/18: 33.3%). The immune protection indices of the vaccine also differed (AH/1807: 94.1, DH/18: 61.1). Additionally, while both strains caused interferon-β and interferon-γ expression to decline, DH/18 infection caused stronger immunosuppression than AH/1807. This inhibition persisted even after vaccination, leading to increased replication of DH/18 that ultimately broke through vaccine immune protection. These results indicate that both strains have different characteristics, and that strains such as DH/18, which cause weaker pathogenic damage but can break through vaccine immune protection, require further attention. Our findings increase the understanding of the differences between epidemic strains and factors underlying MD vaccination failure in China.
Keywords: IFN-β; IFN-γ; Marek’s disease virus; immunosuppression; pathogenicity; vaccination.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Emerging natural recombinant Marek's disease virus between vaccine and virulence strains and their pathogenicity.Transbound Emerg Dis. 2022 Sep;69(5):e1702-e1709. doi: 10.1111/tbed.14506. Epub 2022 Mar 22. Transbound Emerg Dis. 2022. PMID: 35266322
-
Pathogenic characteristics of Marek's disease virus field strains prevalent in China and the effectiveness of existing vaccines against them.Vet Microbiol. 2015 May 15;177(1-2):62-8. doi: 10.1016/j.vetmic.2014.12.020. Epub 2015 Jan 2. Vet Microbiol. 2015. PMID: 25770895
-
Host genetic resistance to Marek's disease sustains protective efficacy of herpesvirus of turkey in both experimental and commercial lines of chickens.Vaccine. 2014 Apr 1;32(16):1820-7. doi: 10.1016/j.vaccine.2014.01.092. Epub 2014 Feb 13. Vaccine. 2014. PMID: 24530405
-
Marek's disease vaccines: a solution for today but a worry for tomorrow?Vaccine. 2008 Jul 18;26 Suppl 3:C31-41. doi: 10.1016/j.vaccine.2008.04.009. Vaccine. 2008. PMID: 18773529 Review.
-
Vaccinal control of Marek's disease: current challenges, and future strategies to maximize protection.Vet Immunol Immunopathol. 2006 Jul 15;112(1-2):78-86. doi: 10.1016/j.vetimm.2006.03.014. Epub 2006 May 8. Vet Immunol Immunopathol. 2006. PMID: 16682084 Review.
Cited by
-
Effect of MHC Haplotype on Mortality Due to Marek's Disease in Commercial Laying Hens.Animals (Basel). 2025 Jun 3;15(11):1647. doi: 10.3390/ani15111647. Animals (Basel). 2025. PMID: 40509113 Free PMC article.
-
Pathogenicity and transmissibility of Marek's disease virus isolated from chickens in Thailand.Poult Sci. 2025 Jul 3;104(10):105519. doi: 10.1016/j.psj.2025.105519. Online ahead of print. Poult Sci. 2025. PMID: 40633314 Free PMC article.
-
Application of lentinan in suppression of Marek's disease virus infection.Poult Sci. 2024 Dec;103(12):104427. doi: 10.1016/j.psj.2024.104427. Epub 2024 Oct 13. Poult Sci. 2024. PMID: 39490132 Free PMC article.
References
-
- Gimeno I.M., Cortes A.L., Faiz N.M., Hernandez-Ortiz B.A., Guy J.S., Hunt H.D., Silva R.F. Evaluation of the Protection Efficacy of a Serotype 1 Marek’s Disease Virus-Vectored Bivalent Vaccine Against Infectious Laryngotracheitis and Marek’s Disease. Avian Dis. 2015;59:255–262. doi: 10.1637/10966-103014-Reg. - DOI - PubMed
-
- Teng M., Zheng L.-P., Li H.-Z., Ma S.-M., Zhu Z.-J., Chai S.-J., Yao Y., Nair V., Zhang G.-P., Luo J. Pathogenicity and Pathotype Analysis of Henan Isolates of Marek’s Disease Virus Reveal Long-Term Circulation of Highly Virulent MDV Variant in China. Viruses. 2022;14:1651. doi: 10.3390/v14081651. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

