Antiviral Activity of Acetylsalicylic Acid against Bunyamwera Virus in Cell Culture
- PMID: 37112928
- PMCID: PMC10141918
- DOI: 10.3390/v15040948
Antiviral Activity of Acetylsalicylic Acid against Bunyamwera Virus in Cell Culture
Abstract
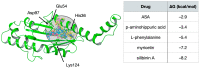
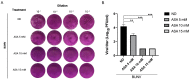
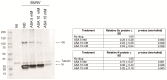
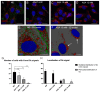
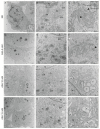
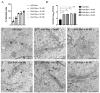
The Bunyavirales order is a large group of RNA viruses that includes important pathogens for humans, animals and plants. With high-throughput screening of clinically tested compounds we have looked for potential inhibitors of the endonuclease domain of a bunyavirus RNA polymerase. From a list of fifteen top candidates, five compounds were selected and their antiviral properties studied with Bunyamwera virus (BUNV), a prototypic bunyavirus widely used for studies about the biology of this group of viruses and to test antivirals. Four compounds (silibinin A, myricetin, L-phenylalanine and p-aminohippuric acid) showed no antiviral activity in BUNV-infected Vero cells. On the contrary, acetylsalicylic acid (ASA) efficiently inhibited BUNV infection with a half maximal inhibitory concentration (IC50) of 2.02 mM. In cell culture supernatants, ASA reduced viral titer up to three logarithmic units. A significant dose-dependent reduction of the expression levels of Gc and N viral proteins was also measured. Immunofluorescence and confocal microscopy showed that ASA protects the Golgi complex from the characteristic BUNV-induced fragmentation in Vero cells. Electron microscopy showed that ASA inhibits the assembly of Golgi-associated BUNV spherules that are the replication organelles of bunyaviruses. As a consequence, the assembly of new viral particles is also significantly reduced. Considering its availability and low cost, the potential usability of ASA to treat bunyavirus infections deserves further investigation.
Keywords: Bunyamwera virus; acetylsalicylic acid (ASA); antiviral; bunyavirus; drug repurposing; electron microscopy; high-throughput screening; molecular modeling; viral RNA polymerase; viral replication organelle.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Digoxin is a potent inhibitor of Bunyamwera virus infection in cell culture.J Gen Virol. 2023 Apr;104(4). doi: 10.1099/jgv.0.001838. J Gen Virol. 2023. PMID: 37010894
-
Light and electron microscopy imaging unveils new aspects of the antiviral capacity of silver nanoparticles in bunyavirus-infected cells.Virus Res. 2021 Sep;302:198444. doi: 10.1016/j.virusres.2021.198444. Epub 2021 May 4. Virus Res. 2021. PMID: 33961898
-
Modulation of Potassium Channels Inhibits Bunyavirus Infection.J Biol Chem. 2016 Feb 12;291(7):3411-22. doi: 10.1074/jbc.M115.692673. Epub 2015 Dec 16. J Biol Chem. 2016. PMID: 26677217 Free PMC article.
-
A Review of Bunyamwera, Batai, and Ngari Viruses: Understudied Orthobunyaviruses With Potential One Health Implications.Front Vet Sci. 2018 Apr 12;5:69. doi: 10.3389/fvets.2018.00069. eCollection 2018. Front Vet Sci. 2018. PMID: 29707545 Free PMC article. Review.
-
An Overview of the Infectious Cycle of Bunyaviruses.Viruses. 2022 Sep 28;14(10):2139. doi: 10.3390/v14102139. Viruses. 2022. PMID: 36298693 Free PMC article. Review.
Cited by
-
Imaging Bunyavirus Infections by Transmission Electron Microscopy: Conventional Sample Preparation vs High-Pressure Freezing and Freeze-Substitution.Methods Mol Biol. 2024;2824:241-258. doi: 10.1007/978-1-0716-3926-9_16. Methods Mol Biol. 2024. PMID: 39039417
-
The 125th Anniversary of Aspirin-The Story Continues.Pharmaceuticals (Basel). 2024 Mar 28;17(4):437. doi: 10.3390/ph17040437. Pharmaceuticals (Basel). 2024. PMID: 38675399 Free PMC article. Review.
References
-
- WHO Prioritizing Diseases for Research and Development in Emergency Contexts. [(accessed on 5 April 2023)]. Available online: https://www.who.int/activities/prioritizing-diseases-for-research-and-de....
-
- Jayk Bernal A., Gomes da Silva M.M., Musungaie D.B., Kovalchuk E., Gonzalez A., Delos Reyes V., Martin-Quiros A., Caraco Y., Williams-Diaz A., Brown M.L., et al. Molnupiravir for Oral Treatment of COVID-19 in Nonhospitalized Patients. N. Engl. J. Med. 2022;386:509–520. doi: 10.1056/NEJMoa2116044. - DOI - PMC - PubMed
-
- Schafer A., Martinez D.R., Won J.J., Meganck R.M., Moreira F.R., Brown A.J., Gully K.L., Zweigart M.R., Conrad W.S., May S.R., et al. Therapeutic treatment with an oral prodrug of the remdesivir parental nucleoside is protective against SARS-CoV-2 pathogenesis in mice. Sci. Transl. Med. 2022;14:eabm3410. doi: 10.1126/scitranslmed.abm3410. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

