Nationwide Laboratory Surveillance of Progressive Multifocal Leukoencephalopathy in Japan: Fiscal Years 2011-2020
- PMID: 37112948
- PMCID: PMC10144269
- DOI: 10.3390/v15040968
Nationwide Laboratory Surveillance of Progressive Multifocal Leukoencephalopathy in Japan: Fiscal Years 2011-2020
Abstract
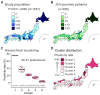
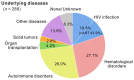
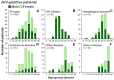
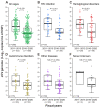
Progressive multifocal leukoencephalopathy (PML) is a devastating demyelinating disease caused by JC virus (JCV), predominantly affecting patients with impaired cellular immunity. PML is a non-reportable disease with a few exceptions, making national surveillance difficult. In Japan, polymerase chain reaction (PCR) testing for JCV in the cerebrospinal fluid (CSF) is performed at the National Institute of Infectious Diseases to support PML diagnosis. To clarify the overall profile of PML in Japan, patient data provided at the time of CSF-JCV testing over 10 years (FY2011-2020) were analyzed. PCR testing for 1537 new suspected PML cases was conducted, and 288 (18.7%) patients tested positive for CSF-JCV. An analysis of the clinical information on all individuals tested revealed characteristics of PML cases, including the geographic distribution, age and sex patterns, and CSF-JCV-positivity rates among the study subjects for each type of underlying condition. During the last five years of the study period, a surveillance system utilizing ultrasensitive PCR testing and widespread clinical attention to PML led to the detection of CSF-JCV in the earlier stages of the disease. The results of this study will provide valuable information not only for PML diagnosis, but also for the treatment of PML-predisposing conditions.
Keywords: JC virus; cerebrospinal fluid; laboratory surveillance; progressive multifocal leukoencephalopathy; real-time PCR testing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Walker P.J., Siddell S.G., Lefkowitz E.J., Mushegian A.R., Adriaenssens E.M., Alfenas-Zerbini P., Dempsey D.M., Dutilh B.E., García M.L., Curtis Hendrickson R., et al. Recent Changes to Virus Taxonomy Ratified by the International Committee on Taxonomy of Viruses (2022) Arch. Virol. 2022;167:2429–2440. doi: 10.1007/s00705-022-05516-5. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

