A Virus Genetic System to Analyze the Fusogenicity of Human Cytomegalovirus Glycoprotein B Variants
- PMID: 37112959
- PMCID: PMC10142178
- DOI: 10.3390/v15040979
A Virus Genetic System to Analyze the Fusogenicity of Human Cytomegalovirus Glycoprotein B Variants
Abstract
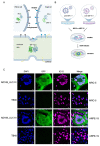
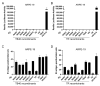
Viruses can induce the fusion of infected and neighboring cells, leading to the formation of syncytia. Cell-cell fusion is mediated by viral fusion proteins on the plasma membrane of infected cells that interact with cellular receptors on neighboring cells. Viruses use this mechanism to spread rapidly to adjacent cells or escape host immunity. For some viruses, syncytium formation is a hallmark of infection and a known pathogenicity factor. For others, the role of syncytium formation in viral dissemination and pathogenicity remains poorly understood. Human cytomegalovirus (HCMV) is an important cause of morbidity and mortality in transplant patients and the leading cause of congenital infections. Clinical HCMV isolates have broad cell tropism but differ in their ability to induce cell-cell fusions, and little is known about the molecular determinants. We developed a system to analyze HCMV glycoprotein B (gB) variants in a defined genetic background. HCMV strains TB40/E and TR were used as vectors to compare the fusogenicity of six gB variants from congenitally infected fetuses with those from three laboratory strains. Five of them conferred the ability to induce the fusion of MRC-5 human embryonic lung fibroblasts to one or both backbone strains, as determined by a split GFP-luciferase reporter system. The same gB variants were not sufficient to induce syncytia in infected ARPE-19 epithelial cells, suggesting that additional factors are involved. The system described here allows a systematic comparison of the fusogenicity of viral envelope glycoproteins and may help to clarify whether fusion-promoting variants are associated with increased pathogenicity.
Keywords: UL55; cell–cell fusion; entry; glycoprotein B; human herpesvirus 5; infectivity.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures






Similar articles
-
Human cytomegalovirus glycoprotein variants governing viral tropism and syncytium formation in epithelial cells and macrophages.J Virol. 2024 Jul 23;98(7):e0029324. doi: 10.1128/jvi.00293-24. Epub 2024 Jun 5. J Virol. 2024. PMID: 38837351 Free PMC article.
-
Human cytomegalovirus glycoprotein B variants affect viral entry, cell fusion, and genome stability.Proc Natl Acad Sci U S A. 2019 Sep 3;116(36):18021-18030. doi: 10.1073/pnas.1907447116. Epub 2019 Aug 19. Proc Natl Acad Sci U S A. 2019. PMID: 31427511 Free PMC article.
-
Monoclonal Antibodies to Different Components of the Human Cytomegalovirus (HCMV) Pentamer gH/gL/pUL128L and Trimer gH/gL/gO as well as Antibodies Elicited during Primary HCMV Infection Prevent Epithelial Cell Syncytium Formation.J Virol. 2016 Jun 24;90(14):6216-6223. doi: 10.1128/JVI.00121-16. Print 2016 Jul 15. J Virol. 2016. PMID: 27122579 Free PMC article.
-
Cell Fusion and Syncytium Formation in Betaherpesvirus Infection.Viruses. 2021 Sep 30;13(10):1973. doi: 10.3390/v13101973. Viruses. 2021. PMID: 34696402 Free PMC article. Review.
-
The COMPLEXity in herpesvirus entry.Curr Opin Virol. 2017 Jun;24:97-104. doi: 10.1016/j.coviro.2017.04.006. Epub 2017 May 21. Curr Opin Virol. 2017. PMID: 28538165 Free PMC article. Review.
Cited by
-
Subconfluent ARPE-19 Cells Display Mesenchymal Cell-State Characteristics and Behave like Fibroblasts, Rather Than Epithelial Cells, in Experimental HCMV Infection Studies.Viruses. 2023 Dec 28;16(1):49. doi: 10.3390/v16010049. Viruses. 2023. PMID: 38257749 Free PMC article.
-
Human cytomegalovirus glycoprotein variants governing viral tropism and syncytium formation in epithelial cells and macrophages.J Virol. 2024 Jul 23;98(7):e0029324. doi: 10.1128/jvi.00293-24. Epub 2024 Jun 5. J Virol. 2024. PMID: 38837351 Free PMC article.
References
-
- Ambrosini A.E., Enquist L.W. Cell-fusion events induced by α-herpesviruses. Future Virol. 2015;10:185–200. doi: 10.2217/fvl.14.100. - DOI
-
- Mohl B.S., Chen J., Longnecker R. Gammaherpesvirus entry and fusion: A tale how two human pathogenic viruses enter their host cells. Adv. Virus Res. 2019;104:313–343. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

