The Flavonoid Cyanidin Shows Immunomodulatory and Broad-Spectrum Antiviral Properties, Including SARS-CoV-2
- PMID: 37112969
- PMCID: PMC10143848
- DOI: 10.3390/v15040989
The Flavonoid Cyanidin Shows Immunomodulatory and Broad-Spectrum Antiviral Properties, Including SARS-CoV-2
Abstract
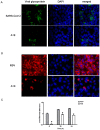
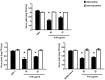
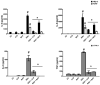
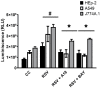
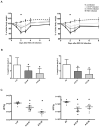
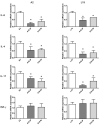
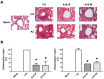
New antiviral treatments are needed to deal with the unpredictable emergence of viruses. Furthermore, vaccines and antivirals are only available for just a few viral infections, and antiviral drug resistance is an increasing concern. Cyanidin (a natural product also called A18), a key flavonoid that is present in red berries and other fruits, attenuates the development of several diseases, through its anti-inflammatory effects. Regarding its mechanism of action, A18 was identified as an IL-17A inhibitor, resulting in the attenuation of IL-17A signaling and associated diseases in mice. Importantly, A18 also inhibits the NF-κB signaling pathway in different cell types and conditions in vitro and in vivo. In this study, we report that A18 restricts RSV, HSV-1, canine coronavirus, and SARS-CoV-2 multiplication, indicating a broad-spectrum antiviral activity. We also found that A18 can control cytokine and NF-κB induction in RSV-infected cells independently of its antiviral activity. Furthermore, in mice infected with RSV, A18 not only significantly reduces viral titers in the lungs, but also diminishes lung injury. Thus, these results provide evidence that A18 could be used as a broad-spectrum antiviral and may contribute to the development of novel therapeutic targets to control these viral infections and pathogenesis.
Keywords: A18; SARS-CoV-2; antiviral; broad-spectrum; cyanidin; immunomodulatory.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Aesculus hippocastanum extract and the main bioactive constituent β-escin as antivirals agents against coronaviruses, including SARS-CoV-2.Sci Rep. 2024 Mar 17;14(1):6418. doi: 10.1038/s41598-024-56759-y. Sci Rep. 2024. PMID: 38494515 Free PMC article.
-
Effect of Jinzhen granule on two coronaviruses: The novel SARS-CoV-2 and the HCoV-229E and the evidences for their mechanisms of action.Phytomedicine. 2022 Jan;95:153874. doi: 10.1016/j.phymed.2021.153874. Epub 2021 Dec 11. Phytomedicine. 2022. PMID: 34923232 Free PMC article.
-
Liu Shen Wan inhibits influenza a virus and excessive virus-induced inflammatory response via suppression of TLR4/NF-κB signaling pathway in vitro and in vivo.J Ethnopharmacol. 2020 Apr 24;252:112584. doi: 10.1016/j.jep.2020.112584. Epub 2020 Jan 21. J Ethnopharmacol. 2020. PMID: 31972325
-
Current status of antivirals and druggable targets of SARS CoV-2 and other human pathogenic coronaviruses.Drug Resist Updat. 2020 Dec;53:100721. doi: 10.1016/j.drup.2020.100721. Epub 2020 Aug 26. Drug Resist Updat. 2020. PMID: 33132205 Free PMC article. Review.
-
Approaches and advances in the development of potential therapeutic targets and antiviral agents for the management of SARS-CoV-2 infection.Eur J Pharmacol. 2020 Oct 15;885:173450. doi: 10.1016/j.ejphar.2020.173450. Epub 2020 Jul 31. Eur J Pharmacol. 2020. PMID: 32739174 Free PMC article. Review.
Cited by
-
Potential of Flavonoids as Promising Phytotherapeutic Agents to Combat Multidrug-Resistant Infections.Curr Pharm Biotechnol. 2024;25(13):1664-1692. doi: 10.2174/0113892010271172231108190233. Curr Pharm Biotechnol. 2024. PMID: 38031767 Review.
-
Imiquimod, a Promising Broad-Spectrum Antiviral, Prevents SARS-CoV-2 and Canine Coronavirus Multiplication Through the MAPK/ERK Signaling Pathway.Viruses. 2025 May 31;17(6):801. doi: 10.3390/v17060801. Viruses. 2025. PMID: 40573392 Free PMC article.
-
Flavonoids in the Treatment of Non-small Cell Lung Cancer via Immunomodulation: Progress to Date.Mol Diagn Ther. 2025 May;29(3):307-327. doi: 10.1007/s40291-025-00772-y. Epub 2025 Mar 4. Mol Diagn Ther. 2025. PMID: 40036006 Review.
-
Aesculus hippocastanum extract and the main bioactive constituent β-escin as antivirals agents against coronaviruses, including SARS-CoV-2.Sci Rep. 2024 Mar 17;14(1):6418. doi: 10.1038/s41598-024-56759-y. Sci Rep. 2024. PMID: 38494515 Free PMC article.
-
Flavonoids with Anti-Herpes Simplex Virus Properties: Deciphering Their Mechanisms in Disrupting the Viral Life Cycle.Viruses. 2023 Nov 29;15(12):2340. doi: 10.3390/v15122340. Viruses. 2023. PMID: 38140581 Free PMC article. Review.
References
-
- Mason S., Devincenzo J.P., Toovey S., Wu J.Z., Whitley R.J. Comparison of antiviral resistance across acute and chronic viral infections. Antivir. Res. 2018;158:103–112. - PubMed
-
- Maarifi G., Martin M.F., Zebboudj A., Boulay A., Nouaux P., Fernandez J., Lagisquet J., Garcin D., Gaudin R., Arhel N.J., et al. Identifying enhancers of innate immune signaling as broad-spectrum antivirals active against emerging viruses. Cell Chem. Biol. 2022;29:1113–1125.e6. doi: 10.1016/j.chembiol.2022.05.009. - DOI - PMC - PubMed
-
- Pasquero S., Gugliesi F., Griffante G., Dell’Oste V., Biolatti M., Albano C., Bajetto G., Delbue S., Signorini L., Dolci M., et al. Novel antiviral activity of PAD inhibitors against human beta-coronaviruses HCoV-OC43 and SARS-CoV-2. Antivir. Res. 2022;200:105278. doi: 10.1016/j.antiviral.2022.105278. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

