HIV-1 Drug Resistance among Treatment-Naïve Patients in Russia: Analysis of the National Database, 2006-2022
- PMID: 37112971
- PMCID: PMC10141655
- DOI: 10.3390/v15040991
HIV-1 Drug Resistance among Treatment-Naïve Patients in Russia: Analysis of the National Database, 2006-2022
Abstract
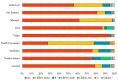
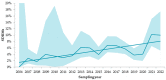
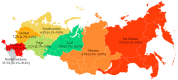
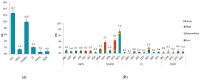
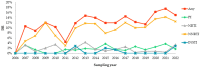
In Russia, antiretroviral therapy (ART) coverage has significantly increased, which, in the absence of routine genotyping testing, could lead to an increase in HIV drug resistance (DR). The aim of this study was to investigate the patterns and temporal trends in HIV DR as well as the prevalence of genetic variants in treatment-naïve patients from 2006 to 2022, using data from the Russian database (4481 protease and reverse transcriptase and 844 integrase gene sequences). HIV genetic variants, and DR and DR mutations (DRMs) were determined using the Stanford Database. The analysis showed high viral diversity, with the predominance of A6 (78.4%), which was the most common in all transmission risk groups. The overall prevalence of surveillance DRMs (SDRMs) was 5.4%, and it reached 10.0% in 2022. Most patients harbored NNRTI SDRMs (3.3%). The prevalence of SDRMs was highest in the Ural (7.9%). Male gender and the CRF63_02A6 variant were association factors with SDRMs. The overall prevalence of DR was 12.7% and increased over time, primarily due to NNRTIs. Because baseline HIV genotyping is unavailable in Russia, it is necessary to conduct surveillance of HIV DR due to the increased ART coverage and DR prevalence. Centralized collection and unified analysis of all received genotypes in the national database can help in understanding the patterns and trends in DR to improve treatment protocols and increase the effectiveness of ART. Moreover, using the national database can help identify regions or transmission risk groups with a high prevalence of HIV DR for epidemiological measures to prevent the spread of HIV DR in the country.
Keywords: HIV-1; Russia; database; drug resistance; treatment-naïve.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Prevalence of HIV-1 drug resistance in Eastern European and Central Asian countries.PLoS One. 2022 Jan 21;17(1):e0257731. doi: 10.1371/journal.pone.0257731. eCollection 2022. PLoS One. 2022. PMID: 35061671 Free PMC article.
-
Temporal Trends in HIV-1 Mutations Used for the Surveillance of Transmitted Drug Resistance.Viruses. 2021 May 11;13(5):879. doi: 10.3390/v13050879. Viruses. 2021. PMID: 34064774 Free PMC article.
-
Transmitted HIV Drug Resistance in Bulgaria Occurs in Clusters of Individuals from Different Transmission Groups and Various Subtypes (2012-2020).Viruses. 2023 Apr 10;15(4):941. doi: 10.3390/v15040941. Viruses. 2023. PMID: 37112921 Free PMC article.
-
Study of the impact of HIV genotypic drug resistance testing on therapy efficacy.Verh K Acad Geneeskd Belg. 2001;63(5):447-73. Verh K Acad Geneeskd Belg. 2001. PMID: 11813503 Review.
-
Transmitted drug resistance among antiretroviral-naive patients with established HIV type 1 infection in Santo Domingo, Dominican Republic and review of the Latin American and Caribbean literature.AIDS Res Hum Retroviruses. 2012 Jul;28(7):667-74. doi: 10.1089/aid.2010.0355. Epub 2011 Sep 23. AIDS Res Hum Retroviruses. 2012. PMID: 21851324 Free PMC article. Review.
Cited by
-
Features of Tat Protein in HIV-1 Sub-Subtype A6 Variants Circulating in the Moscow Region, Russia.Viruses. 2023 Nov 4;15(11):2212. doi: 10.3390/v15112212. Viruses. 2023. PMID: 38005889 Free PMC article.
-
Current Trends of HIV Infection in the Russian Federation.Viruses. 2023 Oct 26;15(11):2156. doi: 10.3390/v15112156. Viruses. 2023. PMID: 38005834 Free PMC article.
References
-
- European Centre for Disease Prevention and Control/WHO Regional Office for Europe . HIV/AIDS Surveillance in Europe 2021–2020 Data. ECDC; Solna, Sweden: 2021. [(accessed on 1 December 2022)]. Available online: https://www.ecdc.europa.eu/en/publications-data/hiv-aids-surveillance-eu....
-
- Ladnaia N.N., Pokrovsky V.V., Sokolova E.V., Chekryzhova D.G., Kirzhanova V.V. Prevalence of human immune deficiency virus infection in the territories of the Russian Federation in 2021. Epidemiol. Infect. Dis. Curr. Items. 2022;12:12–18. doi: 10.18565/epidem.2022.12.3.12-8. (In Russian) - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

