Cellular Immune Responses to SARS-CoV-2 in Exposed Seronegative Individuals
- PMID: 37112977
- PMCID: PMC10144856
- DOI: 10.3390/v15040996
Cellular Immune Responses to SARS-CoV-2 in Exposed Seronegative Individuals
Abstract
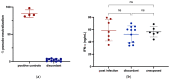
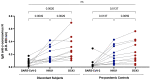
Some SARS-CoV-2-exposed individuals develop immunity without overt infection. We identified 11 individuals who were negative by nucleic acid testing during prolonged close contact and with no serological diagnosis of infection. As this could reflect natural immunity, cross-reactive immunity from previous coronavirus exposure, abortive infection due to de novo immune responses, or other factors, our objective was to characterize immunity against SARS-CoV-2 in these individuals. Blood was processed into plasma and peripheral blood mononuclear cells (PBMC) and screened for IgG, IgA, and IgM antibodies (Ab) against SARS-CoV-2 and common β-coronaviruses OC43 and HKU1. Receptor blocking activity and interferon-alpha (IFN-α) in plasma were also measured. Circulating T cells against SARS-CoV-2 were enumerated and CD4+ and CD8+ T cell responses discriminated after in vitro stimulation. Exposed uninfected individuals were seronegative against SARS-CoV-2 spike (S) and selectively reactive against OC43 nucleocapsid protein (N), suggesting common β-coronavirus exposure induced Ab cross-reactive against SARS-CoV-2 N. There was no evidence of protection from circulating angiotensin-converting enzyme (ACE2) or IFN-α. Six individuals had T cell responses against SARS-CoV-2, with four involving CD4+ and CD8+ T cells. We found no evidence of protection from SARS-CoV-2 through innate immunity or immunity induced by common β-coronaviruses. Cellular immune responses against SARS-CoV-2 were associated with time since exposure, suggesting that rapid cellular responses may contain SARS-CoV-2 infection below the thresholds required for a humoral response.
Keywords: HKU1; OC43; SARS-CoV-2; cellular immunity; cross-reactivity; exposed uninfected.
Conflict of interest statement
The authors declare that no conflict of interest exists in relation to this study. The funders had no role in the design of this study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


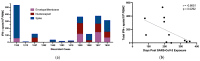
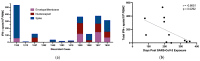

Similar articles
-
Silent battles: immune responses in asymptomatic SARS-CoV-2 infection.Cell Mol Immunol. 2024 Feb;21(2):159-170. doi: 10.1038/s41423-024-01127-z. Epub 2024 Jan 15. Cell Mol Immunol. 2024. PMID: 38221577 Free PMC article. Review.
-
Functional SARS-CoV-2 cross-reactive CD4+ T cells established in early childhood decline with age.Proc Natl Acad Sci U S A. 2023 Mar 21;120(12):e2220320120. doi: 10.1073/pnas.2220320120. Epub 2023 Mar 14. Proc Natl Acad Sci U S A. 2023. PMID: 36917669 Free PMC article.
-
Characterization of SARS-CoV-2-Specific Humoral and Cellular Immune Responses Induced by Inactivated COVID-19 Vaccines in a Real-World Setting.Front Immunol. 2021 Dec 22;12:802858. doi: 10.3389/fimmu.2021.802858. eCollection 2021. Front Immunol. 2021. PMID: 35003131 Free PMC article.
-
Immune responses of lung transplant recipients against SARS-CoV-2 and common respiratory coronaviruses: Evidence for pre-existing cross-reactive immunity.Transpl Immunol. 2023 Dec;81:101940. doi: 10.1016/j.trim.2023.101940. Epub 2023 Oct 20. Transpl Immunol. 2023. PMID: 37866672 Free PMC article.
-
Can T Cells Abort SARS-CoV-2 and Other Viral Infections?Int J Mol Sci. 2023 Feb 22;24(5):4371. doi: 10.3390/ijms24054371. Int J Mol Sci. 2023. PMID: 36901802 Free PMC article. Review.
Cited by
-
Nine-Month Trend of IgG Antibody Persistence and Associated Symptoms Post-SARS-CoV-2 Infection.Healthcare (Basel). 2024 May 5;12(9):948. doi: 10.3390/healthcare12090948. Healthcare (Basel). 2024. PMID: 38727505 Free PMC article.
-
Compartmentalised mucosal and blood immunity to SARS-CoV-2 is associated with high seroprevalence before the Delta wave in Africa.Commun Med (Lond). 2025 May 16;5(1):178. doi: 10.1038/s43856-025-00902-x. Commun Med (Lond). 2025. PMID: 40379979 Free PMC article.
-
Potential protective role of interferon-induced protein with tetratricopeptide repeats 3 (IFIT3) in COVID-19.Front Cell Infect Microbiol. 2024 Nov 27;14:1464581. doi: 10.3389/fcimb.2024.1464581. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39664492 Free PMC article.
-
Silent battles: immune responses in asymptomatic SARS-CoV-2 infection.Cell Mol Immunol. 2024 Feb;21(2):159-170. doi: 10.1038/s41423-024-01127-z. Epub 2024 Jan 15. Cell Mol Immunol. 2024. PMID: 38221577 Free PMC article. Review.
References
-
- Heller T., Werner J.M., Rahman F., Mizukoshi E., Sobao Y., Gordon A.M., Sheets A., Sherker A.H., Kessler E., Bean K.S., et al. Occupational exposure to hepatitis C virus: Early T-cell responses in the absence of seroconversion in a longitudinal cohort study. J. Infect. Dis. 2013;208:1020–1025. doi: 10.1093/infdis/jit270. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

